Benefits of the Randox 5th Generation Bile Acids Assay

Superior methodology
–
Utilising the advanced enzyme cycling method, the Randox 5th generation bile acids assay displayed outstanding sensitivity and precision when compared to the traditional enzymatic based tests.

Excellent measuring range
–
The Randox 5th generation bile acids assay has a measuring range of 2.16 – 238µmol/l for the comfortable detection of clinically important results.

Exceptional correlation
–
A correlation coefficient of r=0.99 was displayed when the Randox method was compared against other commercially available methods.

Liquid ready-to-use
–
The Randox 5th generation bile acids is available in a liquid ready-to-use format for convenience and ease-of-use.

Calibrator and controls available
–
Calibrator and controls are available offering a complete testing package.

Applications available
–
Applications available detailing instrument-specific settings for the convenient use of the Randox 5th generation bile acids assay on a variety of clinical chemistry analysers.
A 4th generation method for bile acids testing is also available which offers an excellent linearity up to 150µmol/l. Applications available detailing instrument-specific settings for the convenient use of the Randox 4th generation bile acids assay on a variety of clinical chemistry analysers.
| Cat No | Size | ||||
|---|---|---|---|---|---|
| BI7982 | R1 6 x 50ml R2 6 x 18ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| BI3863 | R1 2 x 18ml R2 2 x 8ml | Enquire | Kit Insert Request | MSDS | Buy Online |
Instrument Specific Applications (ISA’s) are available for a wide range of biochemistry analysers. Contact us to enquire about your specific analyser.
Intrahepatic cholestasis of pregnancy (ICP) or obstetric cholestasis is a pregnancy-specific liver disorder. ICP, characterised by maternal pruritus in the absence of a rash and increased total bile acids (TBA) levels, is a severe form, yet reversible, cholestasis commonly occurring in the second and third trimester of pregnancy. Diagnostic and therapeutic guidelines are lacking for ICP which is of concern as ICP can have significant foetal risks 1, 2.
ICP restricts the flow of bile through the gallbladder causing bile acids to build-up in the liver 2. Due to the build-up, bile acids leak into the bloodstream where they are detected at concerning levels. It has been documented that TBA levels in ICP can reach as high as 100 times the upper limit of a normal pregnancy. Moreover, a doubling in maternal serum TBA levels, results in a 200% increased risk of stillbirth. Additionally, elevated serum bile acids can affect the foetal cardiovascular system causing issues such as cardiac rhythm disturbances 3.
Whilst other liver function tests exist, including: alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT) and bilirubin; TBA testing is thought to be the most beneficial method for the diagnosis and monitoring ICP. Moreover, TBA measurements are believed to be the most beneficial when tested in conjunction with standard liver tests, offering unrivalled sensitivity enabling the identification of early stage hepatic dysfunction 3.
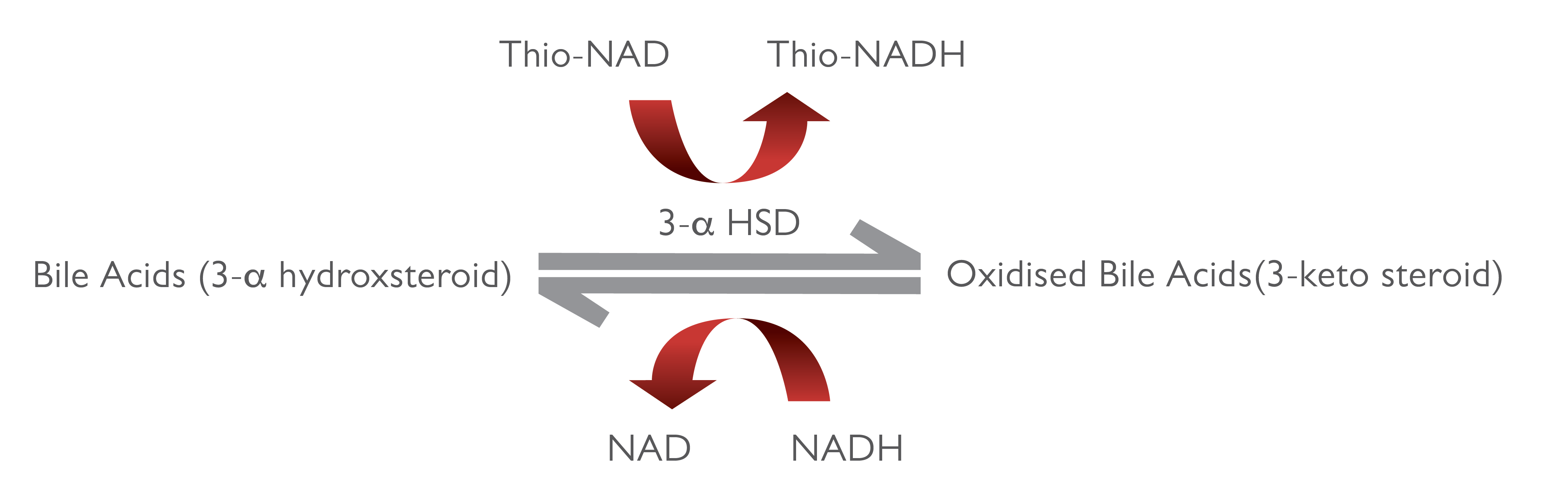
The enzyme cycling method enables signal amplification through cycled regeneration reactions. In the presence of Thio-NAD, the enzyme 3-α hydroxysteroid dehydrogenase (3-α HSD) converts bile acids to 3-keto steroids and Thio-NADH (Fig. 1). The reaction is reversible and 3-α HSD can convert 3-keto steroids and Thio-NADH to bile acids and Thio-NAD. In the presence of excess NADH, the enzyme cycling occurs efficiently and the rate of formation of Thio-NADH is determined by measuring specific change of absorbance at 405nm 5.
Fig 1: Enzyme cycling assay principle 5
Want to know more?
Contact us or download the Total Bile Acids Whitepaper.

