Protecting Pets from the Threat of Mycotoxins
Protecting Pets from the Threat of Mycotoxins
Pet Food companies worldwide are working towards constantly improving and maximising the quality of their product. The problematic topic of mycotoxin contamination in pet feed is quickly becoming a major cause for concern. This is due to the risk they pose for animal health and with the increasing prevalence of mycotoxins globally the focus is on pet food companies to meet EU and FDA regulations and maximise the quality of their product.
What are Mycotoxins?
Mycotoxins are naturally occurring metabolites that are produced by certain moulds and with the ability to develop and grow on a variety of crops they can affect large amounts of feed and increasingly, pet food. If a sample tests positive even for low levels of contamination the toxins are still strong enough to cause illness in animals, and if low levels are consumed over a long period of time this can result in chronic illnesses including; cancer, organ damage and neurological disorders.
The main mycotoxins of concern in pet food are;
- Deoxynivalenol (DON)
- Fumonisins (FUM)
- Zearalenone (ZEN)
- Aflatoxins
- Ochratoxin
- T-2 Toxin
Contamination can occur in any country around the world and at any stage of production. Herein lies the issue of how to prevent mycotoxin pollution, to tackle the issue head on and work towards a mycotoxin free product is the joint responsibility of feed producers, supply chain partners and quality control laboratories ensuring the complete safety of the product.
How can you tell if an animal has ingested pet food contaminated with mycotoxins?
In terms of animal health, mycotoxins can cause a variety of problems. Severity and symptoms can vary from animal to animal but general symptoms include; hyperactivity, vomiting, high temperature and loss of coordination. If you suspect your pet has been affected by mycotoxins you must bring them to the vet for immediate treatment.
The European Union currently regulate all the mycotoxins listed above and are subject to maximum or recommended residue limits. In the US, FDA regulations are limited to aflatoxins, DON and fumonisins, see table below for FDA regulations. If mycotoxin levels in feed fail to meet FDA standards, mass amounts of feed may need to be destroyed as grain producers are prohibited from mixing contaminated feed with clean feed to reduce the mycotoxin levels.
| Pets | Mycotoxin | Commodity | Level |
| Immature Animals | Aflatoxins | Corn/ peanut/ other ingredients | 20 ppb |
| Adult Pets | Aflatoxins | Corn/ peanut/ cottonseed meal/ other ingredients | 20 ppb |
| DON | Grain/ grain byproducts, not to exceed 40% of diet | 5 ppm | |
| Fumonisins | Corn/ corn byproducts, not to exceed 50% of the diet | 10 ppm |
How do we tackle the problem?
Safe, reliable screening solutions for different variations of mycotoxins are available that can ensure only mycotoxin free feed is produced. Randox Food Diagnostics have created mycotoxin screening platforms as a response to increased levels of mycotoxins being found in feed globally.
The platforms use patented Biochip Array Technology (BAT) so pet food producers can test for multiple toxins from a single sample. Randox Food Diagnostics have a range of mycotoxin Biochip Arrays available with customised arrays available to suit the specific screening needs of certain producers. Each Biochip format uses a straightforward extraction process with a 50µl sample of feed, available tests include; Fumonisins, Ochratoxin A, Aflatoxin G1/G2, Aflatoxin B1, Paxiline, Ergot Alkaloids, Diacetoxyscirpenol, Deoxynivalenol, T2 Toxin and Zearalenone.
For more information on mycotoxin screening with Randox Food Diagnostics contact info@randoxfooddiagnostics.com
Obesity: the disease, the problems, and the power of prevention
Earlier this year the World Obesity Federation made the stark statement that: “The early diagnosis and treatment of childhood obesity could be considered similar to vaccination.”
Essentially, they want to see this condition treated in the same way as chicken pox, measles and mumps: tackled – in the hope of eradication – by a strategic approach founded on proactive policies and early prevention.
Obesity in children and adolescents has risen tenfold in the last 40 years, according to a recent study by The Lancet. In Britain, one in ten young people aged between 5 and 19 is obese. Worryingly, the prevalence of obesity is actually higher in younger children than older ones.
The WHO first called for obesity to be understood as a disease in 1948, but back then it wasn’t even considered a risk factor for cardiovascular disease. In 1997 the WHO held a special conference on obesity and stated that: “the global epidemic projections for the next decade are so serious that public health action is urgently required.”
Then it was alarmed that the prevalence of men with a BMI greater than 30 was 15% and 16.5% in women. To think that it has now risen dramatically to 67% for men and 57% for women, highlights just how serious a problem obesity poses to society.
The calls for more countries to officially recognise it as a disease is based on the position that obesity meets the definition of a chronic, relapsing, progressive disease that causes organ damage.
Women and men who are obese are 12.5 and 5.2 times (respectively) more likely to develop diabetes than people who are a healthy weight. 90% of people with Type 2 diabetes are obese.
People with diabetes are then at a greater risk of a range of chronic health conditions including cardiovascular disease, blindness, amputation, kidney disease and depression than people without diabetes. Diabetes leads to a two-fold excess risk for cardiovascular disease, and diabetic retinopathy is the leading cause of preventable sight loss among people of working age in England and Wales. About one in twenty people have diabetes, yet people with diabetes account for one quarter to one third of hospital admissions for cardiovascular disease.
According to Government figures released this year, people who have Type 2 diabetes are 28.4% more likely to die early than their peers.
Getting in front of this wave of diabetes will not only bring down the numbers of people affected but also see a positive impact on the numbers of obese people. As with all conditions – the earlier they are identified, the better. To do this, new methods of diagnosis are being developed.
A radical new test for a protein found in our blood called adiponectin can identify pre-diabetes. This is a game-changing diagnostic tool that empowers people with the knowledge that they are at risk, but may be able to avoid it through relatively simple lifestyle changes.
The adiponectin test is available from Randox – both for clinical use and also through our Randox Health clinics. We have developed the most comprehensive health checks available on the market. These are so sensitive that in a range of conditions including diabetes we are able to identify signs of pre-illness. This enables clients to make often simple changes to stay healthy.
We know that prevention works. The NHS carried out a study in 2016 which revealed an average 26% reduction in new cases of Type 2 diabetes in those participating in a diabetes prevention programme, compared with usual care.
To find out more, click here.
For further information please email: randoxpr@randox.com
Focus on Chronic Kidney Disease diagnosis (CKD)
Chronic Kidney Disease (CKD) is a long term condition which involves the progressive loss of kidney function a late diagnosis can result in end stage renal disease requiring kidney dialysis or transplantation. Typically, CKD is result of a combination of other conditions which puts a strain on the kidneys, these conditions can include high blood pressure, diabetes and high cholesterol amongst many other ailments.1
Randox Biosciences are continually researching and developing new tests, targeting various health concerns around the world to improve diagnostics and health worldwide. Recently, our dedicated scientists have developed a new test, utilising our proprietary Biochip Array Technology (BAT) that simultaneously and quantitatively detects multiple early biomarkers associated with kidney damage allowing for earlier intervention and treatment, preventing further kidney damage.
We offer two multiplex Chronic Kidney Arrays as shown below:
Early detection provides those diagnosed with the opportunity to alter their lifestyle in order to improve their kidney and overall health, whether that is through the reduction of salt in their diet, increased physical activity and alcohol limitation.
To find out more about the Chronic Kidney Disease Arrays offered by Biosciences email info@randoxbisociences.com
1 NHS – https://www.nhs.uk/conditions/kidney-disease/
Powering the Evidence Series – Biochip Array Technology
In 2002, Randox invented a worlds first; Biochip Array Technology, instantly changing the landscape of diagnostic testing forever. Biochip Array Technology is a multi-analyte platform which provides an unrivalled increase in patient information per sample. Instead of a patient sample needing to be subdivided for each test result, or in some cases re-collected, Biochip Array Technology offers a diagnostic patient profile with each patient sample.
How does it work?
Biochip Array Technology is a precision multiplex testing platform allowing for the simultaneous quantitative or qualitative detection of a wide range of analytes from a single sample.
The biochip detection system is based on a chemiluminescent reaction. This is the emission of light, without heat, as a result of a chemical reaction. An enzyme is used to catalyse the chemical reaction on the biochip which generates the chemiluminescent signal. The light emitted from the chemiluminescent reaction that takes place in each DTR is simultaneously detected and quantified using a Charge-Coupled Device (CCD) Camera.
Each biochip has up to 49 Discrete Test Regions (DTR). This means that up to 44 tests can be carried out simultaneously. The additional DTR are reserved for internal quality control and visual reference, a unique Biochip Array Technology feature.
How is the technology applied?
With over £250 million invested into Biochip Array Technology research and development, Randox have launched a range of Biochip Array Technology immunoanalysers – The Evidence Series. This includes the Evidence, the Evidence Evolution, the Evidence Investigator and the Evidence MultiSTAT. Each analyser is developed with boundary pushing engineering, designed to make financial, labour and time savings for the end user.
The Evidence Series has truly revolutionised diagnostic testing forever. Offering unrivalled capabilities across all analysers, we truly believe that the Evidence Series range of immunoassay analysers can meet your diagnostic testing capabilities.
For more information on any of the Evidence Series, please visit http://www.randox.com/evidence-series/ or contact us evidenceseries@randox.com.
This Christmas, treat your laboratory to the “gift” of Randox Quality Control
At Christmas time all around the globe, people search for the best gift for their loved ones, something they will really like. At Randox Quality Control, we understand you care for your patients, and your laboratory. This year, show how much you care by treating your lab to a Randox Quality Control.
With over 35 years’ experience in the market, Quality Control is our passion and streamlining QC practice is our forte! Our extensive product offering comprises true third party controls, interlaboratory data management, external quality assessment and calibration verification.
Last year we looked in depth at Acusera – our range of true third party controls. This year, lets add another product to the Christmas wish list in the form of RIQAS, the world’s largest EQA scheme. Don’t wait until Christmas to wake up to a RIQAS programme under your tree – enrol in one of our comprehensive programmes today.
With over 45,000 laboratory participants across 133 countries, our RIQAS portfolio spans 32 comprehensive programmes ranging from Chemistry to Immunoassay, Lipids to Cardiac, Drugs to Serology and much more!
User-friendly, one page per parameter reports are available within 72 hours of the submission deadline. These reports enable at-a-glance performance assessment, ultimately allowing your laboratory to save valuable time. You will also gain access to complimentary multi-instrument and inter-laboratory reports as well as an end-of-cycle report summarising laboratory performance for each cycle and helping to identify progress over time.
Consolidation is key – and with an extensive parameter index available (up to 360 parameters) RIQAS will help you to significantly reduce costs, time and the number of individual programmes required to cover your test menu.
All RIQAS samples are free from interfering preservatives ensuring a commutable matrix that reacts to the test system in the same manner as a patient sample.
Additionally, enrolling in RIQAS isn’t just enrolling in an EQA scheme. Our programmes are accepted by National and International accreditation bodies worldwide and at the end of each cycle, participation in the scheme results in a certificate that can be used to decorate your laboratory all year around – not just at Christmas!
So this Christmas don’t give your laboratory second best, choose RIQAS, and reap the rewards.
To find out more on any of our RIQAS programmes visit our website – http://www.randox.com/riqas-external-quality-assessment/ or email us at acusera@randox.com
Randox Quality Control wish you all Season’s Greetings & a Prosperous New Year!
Meeting ISO 15189:2012 Requirements for Multiple Instruments
Approximately 70% of clinical decisions are based on laboratory test results. Poor laboratory quality can result in unreliable test results ultimately leading to misdiagnosis, inappropriate treatment and may even impact the overall quality of life for the patient. Having multiple instruments can often add to the difficulties faced in labs. The importance of quality medical services is recognised globally with several bodies existing internationally including ISO (International Organisation for Standardisation) who have developed a set of guidelines and quality systems to ensure reliable test results – ISO 15189:2012.
About ISO 15189:2012
ISO 15189:2012 was designed to outline the “requirements for competence and quality that are particular to medical laboratories”. Laboratory competence and quality are critical in patient diagnosis and care to ensure they meet the need of the clinicians & patients. Gaining accreditation to ISO 15189:2012 will assure clinicians employing your services that they will be benefitting from accurate results which have been measured against a consistent standard. You could benefit too from cost savings and enhanced end-user satisfaction.
Gaining Accreditation
ISO 15189:2012 divides the quality requirements of the laboratory into two distinct areas; Internal Quality Control (IQC) and External Quality Assessment (EQA). By combining both you can comprehensively review and monitor the overall performance of your laboratory, including personnel, equipment, and procedures.
A particular requirement of ISO 15189:2012 is:
“Laboratories accredited according to ISO 15189 that have two or more analysers for examinations, should have a defined mechanism for comparison of results across analysers”
How Randox can help labs with multiple instruments?
Randox offers solutions in both IQC and EQA to help your lab meet the ISO 15189 requirements.
RIQAS
Our international EQA scheme is the largest in the world with 45,000 participants in 133 countries.
Multi-Instrument Reports
All RIQAS participants can register up to five separate instruments per programme at no extra cost. Individual reports for each instrument plus a unique multi-instrument report are provided. The multi-instrument report plots the performance of each individual instrument on a single, colour coded Levey-Jennings chart, ensuring instant identification of any differences in instrument performance. Additional sample packs may be ordered as required.
The multi-instrument report includes many of the same statistical features found in the main RIQAS report including; CV%, SDI, RMSDI, %DEV, RM%DEV, Target Score, and RM Target Score.
Acusera 24.7 Live Online
Our stress free QC analysis software is designed to assist in the management of daily QC activities.
Support for multiple instruments
Acusera 24.7 Live Online allows laboratories to conveniently combine multiple instruments as well as analytes and QC lots on a single Levey-Jennings chart, allowing comparative performance assessment and immediate visualisation of any ongoing or emerging trends.
Helping you get accredited
Randox helps you get accredited by offering products from the full spectrum of Quality Control, meaning you never have to look elsewhere. Not all manufactures can offer these features.
To find out more about how we can help you meet ISO 15189 requirements, contact us using the form below.

Cocaine on the Rise
Newly emerged figures from Public Health England have documented that the UK’s current approach to drug treatment has failed to reduce drug related deaths. With UK drug abuse now at an all-time high, 2017 saw a 23% increase in treatment presentations for crack cocaine use, according to The Conversation. An additional article by the Business Insider UK reported that seizures related to cocaine in Britain are now at their highest since 2008.
Crack cocaine is a powerful stimulant designed to temporarily speed up the mind and body. Freebase cocaine (powder cocaine) and crack cocaine (rock form cocaine) can both be smoked to reach the brain quicker, whilst snorting the substance causes a slower effect. A very addictive substance, cocaine is reported to make a user over confident and careless with risks including, breathing and mental health problems, depression and the risk of an overdose related death. When taken in conjunction with alcohol the dangers of cocaine are increased, as the mixture produces the toxic chemical, cocaethylene.
The Conversation highlighted “Cuts to drug treatment budgets are extremely shortsighted. Not only do effective services save lives, they reduce the spread of blood-borne viruses, including HIV. About half of people who inject drugs have hepatitis C. Getting them into treatment is an essential part of plans to eliminate the disease.” At Randox Toxicology we offer the most comprehensive drugs of abuse test menu across multiple matrices. Our DoA ULTRA panel detects up to 20 targeted drugs, offering the largest cross-reactivity profile of over 240 analytes, including Benzoylecgonine/Cocaine. Benzoylecgonine is the most common metabolite measured in urine drug screens to detect cocaine. Using our revolutionary Biochip Array Technology, Randox Toxicology provide cutting-edge multiplex testing capabilities for rapid and accurate drug detection from a single sample.
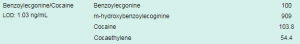
The price of cocaine has fallen by 13% since 2007 according to Business Insider UK. Price trends in addition to new and emerging UK supply routes have made the drug more appealing and readily available. Whilst the average age group using cocaine is 35 years old, a 30% increase has been seen in young people under the age of 25 years old. A rise which has not been witnessed in a decade.
The increase of cocaine use has shown how vital drug treatment is in responding to the ever-changing market, with services needing to adapt quickly to the needs of varied groups. Amidst the ongoing cocaine problem, Randox Toxicology are leading the way in developing new tests through significant research and development.
For further information on how Randox Toxicology are fighting drugs of abuse, email info@randoxtoxicology.com
We Are Randox | John Fitzgerald wins Ulster University awards for Master’s degree project with Randox
Here at Randox, we’re proud of the talented and innovative teams we have at all our sites. One of our talented engineers at Randox Teoranta recently won a prize for his final year university project in collaboration with Randox. John Fitzgerald, an Electronic Design Engineer, was presented with two awards from Ulster University on Thursday 7th December 2017 – the Civica Prize for excellence in his final project and the Institute of Engineering Technology Prize for achieving the highest grades in his class.
We caught up with John to hear all about it;
A very well done on your awards, John! Tell us about your final project for which you won the Civica Prize.
Firstly it is important to note that my final year project was conducted in conjunction with Randox Teoranta. Without the support, resources and encouragement from the exceptional Research & Development Engineering team here in Dungloe, my project would not have been such a success.
My final year project centred on the design of an industry-standard compact dry bath incubator, designed for the heating and cooling of small volume samples. With a simple and compact design, broad and precise temperature range, the intended use of the product was for bench-top laboratory incubations. The design also incorporated innovative, yet modest, capacitive touch pad controls and a digital display to provide confident temperature selection and accuracy.
This design project required design capabilities in three core engineering disciplines, electronic, embedded and mechanical engineering.
Were you surprised to learn you’d won an award for the project?
Yes, definitely! I was surprised when I received an email at the end of November, informing me that I was to receive the award. I can recall the quality of projects that were on show so this was a complete surprise to me.
I invested a great effort in this project and I’m proud of the personal and academic goals I’ve attained, however, the works achieved would not have been possible without the generous investment of advice from various different sources. I wish to take this opportunity to express my genuine appreciation and thanks to them all.
Thank you to Randox – the industrial knowledge and resources they provided for this wrk added significantly to the quality and relevance of my project to the real world. A special word of thanks, too, should also be afforded to my final year supervisor in Ulster University for the consistent academic support he delivered throughout the course of this MEng final year project.
Did you always want to be an engineer?
To be perfectly honest, the answer to this question is no. I was very uncertain for a long time what career I wanted to pursue as a secondary school student. I was never really exposed to the engineering profession and the wide variety of career paths it can lead to so engineering wasn’t something I immediately thought of when I was thinking of careers I would enjoy.
My father has a lot to answer for though – he was a tool-maker by trade and he instilled a significant interest in engineering and basic electronics in me, and is probably one of the primary reasons I felt a career in electronic engineering was the correct path for me. I decided to apply for my university placement year at Randox Teoranta in the Electronic Engineering team.
After just a few months in to my placement at Randox Teoranta, I knew I had made the correct career choice. I was Randox Teoranta R&D Engineering’s first university placement student, and that I could live at home in Donegal for the year and still receive a first class industrial experience.
How did you find your placement year at Randox?
My placement experience at Randox Teoranta was first class. I was afforded every opportunity to develop and grow my engineering skills. As my competency grew, so did my responsibilities and the complexity of jobs afforded to me.
The team of engineers in Randox Teoranta are exceptional professionals and provided excellent guidance to me as a young student engineer. The work I was tasked with was challenging and relevant and a considerable amount of the work I contributed to, remains in some form in the final Misano analyser that is manufactured today in Dungloe.
I cannot stress enough how important my placement year at Randox Teoranta was for me upon returning for my final two years of university. It provided me with a clear career path and I discovered a passion for Printed Circuit Board Design that I would not have been exposed to, if it were not for this placement.
I was extremely grateful to be offered a graduate position during my placement year on completion of my degree. This security made my final two years at university much more comfortable and also allowed me to discuss with the company the potential to complete my final year project in conjunction with Randox Teoranta. The opportunity to continue my learning and professional development as part of such a progressive and diverse engineering environment was an easy decision to make. As an added bonus, I am able to live at home, in the most beautiful part of the country and engage in an extremely rewarding and challenging profession in my field of study all at the same time. I consider myself very fortunate.
Tell us what a typical day is like in your role as Electronic Engineer.
One of the reasons I enjoy being an Electronic Engineer with Randox Teoranta to such a high degree, is the same reason that makes this question quite difficult to answer.
It is hard to categorise a typical day in my role as an Electronic Engineer in Randox Teoranta. I spend my time on a wide variety of duties or tasks depending on the design needs of the engineering team. I could be spending my time designing circuit schematics for new PCB designs, I could be producing the printed circuit board layout of designed circuit schematics, I could be testing new sensors, electronic parts or manufactured PCB’s to verify their performance, I could be engaging in verification and validation work for a new analyser, I could be engaging in the formation of critical design reports, the list can go on and on.
As the cliché goes, “every day is different”, something which is definitely applicable in this scenario.
What advice would you give to young people considering visiting the Randox Teoranta open day on Fri 22nd December?
I would encourage any young person with a remote interest in a career in Science or Engineering to attend the open day on Fri 22nd December. I believe they will be surprised as to the wide variety of professions and opportunities available at their doorstep.
A conversation with an experienced professional could ignite a spark which could provide clarity as to what they would like to pursue in further education, and in turn professionally. This is an opportunity I wish I was afforded as a young person growing up in rural Donegal, and I consider it an opportunity not to be missed for young people with a genuine interest in these exciting professional fields.
From all the staff at Randox, congratulations to John on this fantastic achievement. We look forward to seeing the pioneering engineering work you will continue to be part of in the future.
The Randox Teoranta Open Morning is on Friday 22nd December 2017 from 10am – 2pm at Randox Teoranta, Meenmore, Dungloe, Co. Donegal.
To find out more tel: +353 7495 22600 or email: randoxpr@randox.com
Pictured with John Fitzgerald (centre) is Dr. Robert McMurray, course director for MEng Engineering at Ulster University (left), and Angela Canavan, Managing Director of Civica who was present to award the Civica prize (right).
Mythbusting: Frequency of EQA Reports
External Quality Assessment (EQA) is a vital aspect of laboratory testing and is often a regulatory requirement. The primary function of EQA is to provide the laboratory with an indication of test system accuracy through interlaboratory comparison. Ultimately participation in an EQA programme will give the laboratory greater confidence in the accuracy and reliability of the results they release.
When choosing an EQA scheme there are a variety of different options. It is important that laboratories weigh up the pros and cons of each option in order to choose the scheme that best suits their needs.
Many EQA schemes promote the distribution of multiple EQA samples per challenge or testing event as a benefit to the laboratory. By analysing multiple samples at each testing event, laboratories believe they are challenging their test system across a wider range of concentrations than would be possible if they were to analyse just one sample. However, this is not always the case as in many instances the levels can be very similar. Another consideration is the EQA report received by the laboratory. EQA reports are used to assess analytical performance and identify any test system failures, such reports can take more than one month to receive. In such instances it would be more beneficial if the laboratory tested just one sample at each bi-weekly/monthly challenge and received their report faster.
Report frequency should be priority for any laboratory when choosing an EQA scheme. A fast turnaround time will allow any test system errors to be identified sooner and necessary corrective actions to be taken immediately with minimum disruption to the lab. Perhaps the most important benefits of a rapid report turnaround is cost and time savings as it will significantly reduce the time and money spent re-running patient samples.
If the time between sending EQA results back to the organizer and receiving an EQA report is too long, it becomes more difficult to pin-point exactly when an error was encountered thus increasing the possibility of incorrect test results and possible misdiagnosis. While this would be considered the worst-case scenario, it is still a possibility.
Choosing an EQA provider that delivers both frequent analysis and a quick report turnaround will give the laboratories the confidence they need to report patient test results.
With the Randox International Quality Assessment Scheme (RIQAS), comprehensive reports are available within 72-hours of the submission deadline. Furthermore both bi-weekly and monthly analysis options are available.
To find out more on RIQAS and our extensive product portfolio, comprising 32 programmes, visit our page – http://www.randox.com/riqas-external-quality-assessment/ or alternatively contact us using the button provided.
The Importance of a Workplace Substance Misuse Policy
What is a workplace substance misuse policy?
Many organisations implement a policy that outlines the expectations they have concerning the misuse of drugs and/or alcohol and how this can affect performance and safety in the workplace. This can often be referred to as a substance misuse policy.
Employers hold the responsibility to ensure employees are fully aware of the company’s rules, regulations, testing and disciplinary procedures. Fundamentally, this is to guarantee complete workplace safety. The policy itself holds a vital importance providing employees with the knowledge of the standards expected of them in the workplace.
To ensure transparency of information, it is imperative that the policy is written in a clear, comprehensive manner. This should allow them to understand without confusion or misinterpretation the implications the organisation has in place for users who misuse substances.
Why is this policy so important for businesses to implement?
Alcohol and / or drug use increases the chances of problems occurring in the workplace. For example, studies have found that employees with alcohol problems are 2.7 times more likely to have an accident whilst at work.
Some of the main issues that associated with substance misuse in the workplace are:
- Absenteeism – It’s estimated that there are 17 million lost days of work per year due to substance misuse.
- Low productivity levels – Employees may lose focus in different tasks and become de-motivated.
- Inappropriate behaviour – Some cases of substance abuse may lead to inappropriate actions or in extreme cases crime
Evidence suggests that many people who suffer from alcohol and drug abuse are in employment. Studies show that 25% of registered drug addicts are in full-time employment and that 3.3% of all adults in England and Wales aged 16 – 59 classified as frequent users.
Significant issues such as these provide growing concerns for employers to put in place a workplace substance misuse policy to guarantee the welfare of all stakeholders. Under the Health & Safety Act 1974, employers have a duty to ensure the safety of their employees is met to all principles. Introducing an effective workplace policy is a key part in maintaining these standards.
The importance of a workplace policy for drugs and alcohol can benefit employers by:
- Building relationships with employees by showing them there is support available.
- Policies can raise awareness of issues in the business and can encourage staff members to take action if they feel they have a problem.
- Its importance can reduce the number of sick employees, it can also increase staff turnover & productivity levels.
- Employers who implement a workplace policy can also see a rise in efficiency, improved staff relationship, communication and improved corporate image and in some cases customer relations.
What we at Randox Testing Services can do:
Randox Testing Services is a market leader in the drug and alcohol testing industry. Our expertise is relied upon by safety-critical companies all over the world. We provide complete drug and alcohol testing solutions to a range of blue chip companies across multiple industries including aviation, transport, construction, maritime, retail and occupational health to name a few.
Our expert consultancy service allows employers to create an effective drug and alcohol misuse policy. We offer comprehensive advice, guidance and support in composing an effective policy and explaining best procedures and testing methods to use. We also provide assistance to employers with an existing substance misuse policy, helping to modify existing documents to ensure they are legally viable and can withstand challenge in court.
To ensure clear communication of any policy changes, our experts can come to your workplace and present the policy to your staff. By having a third party explain the policy employers can ensure all the benefits are communicated effectively. This way staff do not feel like they are being victimised or persecuted, but know their employer is looking after their workplace wellbeing.
Combining our consultancy service with our drug and alcohol testing service and / or point of care products, employers can find a complete testing solution from Randox Testing Services.
For more information on our products and services call +44 (0) 28 9445 1011, email, testingservices@randox.com or visit our website www.randoxtestingservices.com
About Randox Testing Services
At Randox Testing Services we offer complete drug and alcohol testing solutions to a wide range of industries across the globe. We work with companies to help create and implement effective workplace testing policies that suit their needs and budget.
To support our customers our additional services include training and education courses as well as policy reviews and consultancy. Our experts are at hand to discuss any testing requirements you may have. To get in touch email testingservices@randox.com today.








