How Randox R&D Scientists are helping to change healthcare: An introduction to diagnostics for BSW 2017
How Randox R&D Scientists are helping to change healthcare: An introduction to diagnostics for BSW 2017
In celebration of British Science Week 2017, we will be giving you an introduction to diagnostics, and exploring how Randox Scientists are helping to change healthcare.
You may or may not already know that Randox are one of the leading diagnostics companies globally. But what exactly does clinical diagnostics involve? It is one of the fundamental steps of finding out what is wrong with a person when they are ill. Read on to find out a bit more about diagnostics, and how the Randox Reagents R&D Scientists are helping to change healthcare globally!
What is a diagnostic test?
A diagnostic test is any kind of analysis performed on a patient sample (a sample is typically blood, urine or cerebrospinal fluid (CSF)), to aid in the diagnosis or detection of disease. The information found from a test can be used to:
- Diagnose disease
- Assess the extent of damage
- Monitor the effectiveness of treatment
- Confirm a person to be free from disease
Examples of substances that may be tested for the blood include proteins, nutrients, waste products, antibodies, hormones, salts, trace elements or vitamins. These are sometimes referred to as ‘analytes’, ‘markers’ or ‘biomarkers’.
This is where reagents come in…
A reagent is a substance which is mixed with the patient sample to create a chemical reaction to detect the biomarker. These reactions are analysed by machines known as analysers.
Finally…
Using data gathered from both clinical symptoms and laboratory tests, the doctor will follow a sometimes painstaking process of analysis and elimination to perform a successful diagnosis!
Randox Reagents celebrate World Kidney Day 2017
On 9 March 2017, Randox Reagents are celebrating World Kidney Day! World Kidney Day is a global campaign aimed at raising awareness of the importance of our kidneys to our overall health. It aims to reduce the frequency and impact of kidney disease and its associated health problems worldwide.
This year, the World Kidney Day promotes education on the harmful consequences of obesity and its association with kidney disease, advocating healthy lifestyle and health policy measures that make preventive behaviours an affordable option.
With this in mind, throughout the week we have been sharing on social media some interesting facts on diagnostic tests which can help aid an early risk assessment of kidney disease in obese patients, allowing preventative action to be taken before any serious damage occurs. The tests of focus this week included cystatin C, adiponectin and microalbumin…
Cystatin C
The creatinine test is routinely run for patients who are suspected for deteriorating kidney function, however this test has limitations. Cystatin C is an alternative test, and is particularly useful in patients where creatinine measurements are not suitable e.g. individuals who are obese, malnourished, have liver cirrhosis or reduced muscle mass. Importantly, unlike creatinine, cystatin C does not have a ‘blind area’ – up to 50% of kidney function can be lost before significant creatinine elevation occurs. Cystatin C is extremely sensitive to very small changes in kidney function and is therefore capable of detecting early stage kidney dysfunction. The cystatin C test therefore allows preventative measures to be taken much earlier and before significant kidney function decline.

Adiponectin
There is substantial evidence that excess visceral fat is the main driving force for almost all of the disorders associated with the metabolic syndrome, including CKD.1,2 The adiponectin test from Randox can accurately assess levels of abdominal visceral fat, independent of age, race or fitness level.3,4 Assessing adiponectin, and therefore visceral fat levels, can help assess risk of CKD, as well as a range of other illnesses such as pre-diabetes, CVD and various cancers.
Microalbumin
The microalbumin test detects very low levels of a blood protein called albumin, in urine. The detection of albumin in urine can be an indicator of kidney injury and can result in irreversible damage if left untreated. Low albumin concentrations in the urine are the earliest marker of kidney damage and therefore enable preventative measures to be taken. Microalbumin testing can identify individuals with diabetic nephropathy approximately 5-10 years earlier than proteinuria tests helping reduce the frequency of end stage renal disease.
Both World Kidney Day and Randox are working towards improving healthcare worldwide. With continuous investment in R&D, Randox are helping with the risk assessment and earliest detection of renal function problems. By assessing one’s risk of kidney problems (with the adiponectin test), it can give patients (obese and other) the tools to prevent kidney problems further on down the line. With early diagnosis (through the cystatin C and microalbumin tests) it will be possible to keep kidney problems from getting worse, therefore lowering the number of those diagnosed with CKD worldwide.
What is Measurement of Uncertainty?
Measurement Uncertainty (MU) relates to the margin of doubt that exists for the result of any measurement, as well as how significant the doubt is. For example, a piece of string may measure 20 cm plus or minus 1 cm, at the 95% confidence level. As a result, this could be written: 20 cm ±1 cm, with a confidence of 95%. Therefore, we are 95% sure that the piece of string is between 19 cm and 21 cm long.
Standards such as ISO 15189 require that the laboratory must determine uncertainty for each test. However, they have not specified how this should be done.
How do we calculate Measurement Uncertainty using QC data?
Employing your QC data to calculate uncertainty makes several assumptions; your test system is under control, the patient samples are treated in the same manner as your controls and gross outliers have been removed. If you choose to use your QC data to calculate this you should ensure that you use a commutable control with a matrix similar to that of a patient sample, with analytes present at clinically relevant levels
To calculate MU, labs must look at the intra-assay precision and inter-assay precision of their test.
Intra-assay precision: Sometimes known as ‘within run’ precision, is where 20 or more replicates of the same sample are run at the same time, under the same conditions (calculated from a single experiment). Intra-assay precision helps to assess systematic uncertainties
Inter-assay precision: Sometimes known as ‘between run’ precision, is where 20 or more replicates are run at different times – e.g. 1 replicate every day for 20 days (can be calculated from routine IQC data). Inter-assay precision can help identify random uncertainties within the test system.
*The Australian Association of Clinical Biochemists (AACB) recommends that at least 6 months’ worth of QC data are used when calculating the inter-assay precision1.
Once the data is collected, you must calculate the standard error of the mean (SEM) of the intra-assay precision (A) and the SD of the inter-assay precision (B) in order to measure the uncertainty (u). Once A and B have been calculated, they need to be squared, added together and the square root of the sum found:
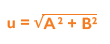
As uncertainty is calculated as SD and 1SD is equal to 68% confidence on a standard Gaussian curve, we can conclude that if we multiply using a coverage factor of 2, we can attain 2SD confidence of 95%. This is known as the Expanded Uncertainty (U):
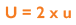
What is the Advantage of Measurement Uncertainty for a lab?
Labs need to carry out MU as it is a requirement of ISO 15189. It states: “The laboratory shall determine measurement uncertainty for each measurement procedure, in the examination phases used to report measured quantity values on patients’ samples. The laboratory shall define the performance requirements for the measurement uncertainty of each measurement procedure and regularly review estimates of measurement uncertainty”.
MU also helps determine whether the difference between two results is negligible due to uncertainty or significant due to a genuine change in condition of the patient; giving labs a greater confidence in reported results.
How can Randox help?
Our new Acusera 24.7 Live Online software provides automatic calculation of MU, saving valuable time and helping labs meet ISO 15189 requirements with ease.
Contact marketing@randox.com to find out how your lab can benefit from Acusera 24.7 Live Online
Take steps to prevent incorrect patient results by making one simple change
According to the NHS Litigation Authority; in 2015 within the UK alone, £193,680,744.30 was spent on ‘wrong diagnosis’ or ‘failed/delayed diagnosis’ causing huge financial strain and impact on labs.
With approximately 75% of clinical decisions and diagnosis based on laboratory test results. The only way to guarantee a high degree of accuracy is to implement a good Quality Control plan. The importance of this is recognised globally, several bodies exist internationally including ISO (International organisation for standardisation) who have developed a set of guidelines and quality systems to ensure the reliability of laboratory test results.
So what can you do to improve accuracy and reliability?
Choose a third party QC
ISO 151589:2012 Section 5.6.2.2 states that “the use of third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer”.
First Party Controls are those manufactured by the instrument/reagent manufacturer. These controls are optimised specifically for use with the manufacturers test system and therefore will mask a multitude of weaknesses. First Party Controls tend to result in perceived accuracy and a biased assessment of performance.
Third Party Controls on the other hand are designed to be completely independent and are not optimised for use with a specific test or system. Leading manufacturers of third party controls will assign target values based on data collected from thousands of independent laboratories, ensuring the availability of statistically robust multi-method, multi-analyser data. Therefore laboratories using Third Party Controls can be assured of unbiased error detection across multiple platforms.
Randox Acusera is a world leading manufacturer of true third party controls providing a cost effective, high quality solution for any laboratory-regardless of size or budget.
Look out for QC samples with clinically relevant concentrations
ISO 15189:2012 states that ‘The laboratory should choose concentrations of control materials wherever possible, especially at or near clinical decision values, which ensure the validity of decisions made’.
It is important to assess the full clinical range of an assay i.e. the range between the lowest and highest results which can be reliably reported. In order to make sure a laboratory instrument is performing accurately across the full clinical range and in particular at the medical decision level, QC materials that cover low, normal and elevated concentrations should be used.
Due to the superior manufacturing process used by Randox, QC target values consistently cover the MDL of tests. By ensuring the controls in use cover clinical decision levels laboratories can be confident of the reliability and accuracy of the patient results they release.
Opt for a commutable control material
A good QC material has many essential properties but above all, controls must perform consistently and reflect the performance of patient samples – if a control meets these requirements then we can say it is commutable. Having a commutable control would aid in the prevention of incorrect patient results because they replicate the performance of a patient sample and react to the test system in a similar manner. Use of a commutable control will also reduce costly shifts in QC target values when reagent batch is changed.
At Randox we take quality seriously, that’s why all QC products are manufactured to the highest possible standard, delivering controls of unrivalled quality. Designed to be commutable, the Acusera range will ensure accurate and reliable instrument performance while simultaneously helping laboratories to meet ISO 15189:2012 requirements. A good QC process will include the use of Third Party Controls, Clinically Relevant Concentrations and controls which can be described as commutable. By employing Quality Control’s that encompass these traits, a laboratory professional can be certain that they have taken the necessary steps to decrease incorrect results and therefore potential misdiagnosis.
Extensive study confirms the benefit of testing apolipoproteins E, C-II and C-III to assess cardiac risk
A study published on 21st February 2017 in the Journal of the American College of Cardiology has found that measuring apolipoproteins E, C-II and C-III can offer earlier detection of cardiovascular risk in comparison to routine apolipoprotein A-I and B tests.1
The lead author of the study, Professor Manuel Mayr, from King’s College London has said, “We directly compared the association of a broad panel of apolipoproteins to new onset of cardiovascular disease over a 10-year observation period, and found that while apoB was predictive, other apolipoproteins, namely apoE, apo C-II and apo C-III, were even better”. Professor Mayr further implied that the findings provide support that expanding current cardiac screening tests to include apolipoproteins could reduce risk of cardiovascular diseases.2
What are apolipoproteins?
Apolipoproteins are proteins that bind to lipids to form lipoproteins. Lipoproteins are made of proteins and fats, and serve the function of transporting insoluble fats, such as cholesterol and triglycerides, to be used by different cells. 3
There are six major types of apolipoprotein: A, B, C, D, E and H and the lipoproteins within these categories can vary in size, density and lipid composition. The study found that apolipoproteins E, C-II and C-III are linked to very low-density lipoproteins (vLDL) and have a stronger association with cardiovascular diseases in comparison to apolipoprotein A-I and apolipoprotein B.4
vLDL is strongly associated with the development of atherosclerosis, the build-up of fatty material inside the arteries, which is a major risk factor of cardiovascular diseases as it can lead to angina, heart attack, stroke or peripheral arterial disease.5
Why measure apo C-II, apo C-III and apo-E?
As highlighted by the authors of the study, cardiovascular risk assessment is commonly associated with only a few lipids within established lipoprotein classes, such as LDL.1 This emphasises the importance of carrying out detailed lipid testing to identify all subgroups to provide a complete cardiovascular risk assessment, as traditional biomarkers for lipids may only provide a limited overview. This can then allow for effective treatment to be provided at an earlier stage, which could subsequently reduce the risk of death by cardiovascular diseases.
Randox offer a range of routine and novel cardiac assays to provide a complete cardiac risk assessment, including: Apolipoprotein C-II / C-III / E / A-I / A-II / B, Adiponectin, HDL Cholesterol, HDL3 Cholesterol, LDL Cholesterol, sLDL Cholesterol, Total Cholesterol, TxBCardio™, H-FABP, Homocysteine, hsCRP, Lipoprotein (a), sPLA2-IIA, and Triglycerides. For more information, email: reagents@randox.com.
References
1. Mayr, M. et al., Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III., Journal of the American College of Cardiology. Vol. 69, No. 7, 2017.
2. NIHR Biomedical Research Centre at Guy’s and St Thomas’ and King’s College London, Discovery could help doctors to spot cardiovascular disease at an earlier stage: Advanced technologies provide researchers with new insights into the warning signs for cardiovascular disease, ScienceDaily (2017) Available from: https://goo.gl/XkC23R [Accessed: 21 February 2017]
3. Kingsbury, K. J., Understanding the Essentials of Blood Lipid Metabolism, Medscape, (2017) Available from: https://goo.gl/AApW6S [Accessed: 23 February 2017]
4. Wallace, A., New technique could aid in earlier diagnosis of heart disease, UPI, (2017) Available from: https://goo.gl/xzxLdf [Accessed: 23 February 2017]
5. British Heart Foundation, Atherosclerosis, (2017) Available from: https://goo.gl/1qHxpk [Accessed: 23 February 2017}

Aliquoting for longer QC stability
Al-i-quot: An amount that is an exact divisor of the whole quantity of a substance (Collins Dictionary of Medicine, R. Young, 2005).
Why aliquot QC material?
Aliquoting QC material can extend the open vial stability of a lyophilised control, according to manufacturer recommendations. By splitting your QC material into a number of tubes and freezing these you can extend the working stability of the control, ultimately reducing wastage and the amount of money spent on unnecessary additional controls.
Example
A laboratory purchases a lyophilised QC with a volume of 3ml once reconstituted the control is stable for 7 days at 2-8oC. However, the laboratory only uses 1ml of this control per week, meaning that 2ml could potentially be wasted. The manufacturer states that the control can be frozen after reconstitution, extending the working stability from 7 days at 2-8oC to 30 days at -20 oC to -80oC. The following outlines the process for aliquoting reconstituted material and extending the control’s working stability.
Aliquoting reconstituted material
- Reconstitute the QC material according to the manufacturer’s instructions.
- Using a micropipette aliquot the required volume (generally a minimum of 0.5ml should be used) of reconstituted material into a tube.
- Repeat step 2 until all the reconstituted material has been aliquoted.
- Label each tube with the date the material was reconstituted to avoid the use of expired material.
- Store each aliquot at -20oC in a frost free freezer. Be sure to check the kit insert for frozen stability claims.
- Remove and thaw each aliquot as and when required making sure to use all material within the frozen stability period.
- Once thawed do not refreeze, dispose of any leftover QC material.
Conclusion
Aliquoting reconstituted material is an ideal way of extending the control’s open vial stability. This will ensure that your laboratory minimises the amount of QC material wasted and saves money by eliminating the need to purchase additional controls. Please note that not all lyophilised controls can be frozen like this. To ensure the controls you are selecting are suitable for aliquoting check the product’s kit insert or contact your supplier.
What can Randox Quality Control offer?
We have a number of lyophilised controls which can be prepared and stored in this way across our extensive product portfolio. To find out more visit www.randoxqc.com or contact us via acusera@randox.com to arrange a visit from one of our QC Consultants.
Inflammatory Biomarker Series: Antioxidants
So far in our inflammatory biomarker series, we have considered the clinical significance of measuring rheumatoid factor (RF) and C-reactive protein (CRP) to detect inflammation. Inflammation, either chronic or acute, is the body’s immune response to protect against harmful stimuli such as damaged cells, irritants or pathogens and can be present in a range of diseases and conditions.1 Measuring inflammatory biomarkers can assist clinicians in the identification of a particular disease or can provide a marker of treatment response. In this blog, we consider the role of antioxidants and identify relevant biomarkers which may be linked to inflammatory states.
What is an antioxidant?
An antioxidant is a molecule that inhibits the oxidation of other molecules. Oxidation is a chemical reaction that produces free radicals, which are groups of very reactive molecules that can interrupt important cellular processes. Antioxidants are commonly referenced with regards to food, however antioxidants are also found in the body in the form of enzymes. Their purpose is to protect against the effects of oxidative stress to reduce damage from free radicals.
What is the link between antioxidants and inflammation?
Oxidative stress and the inflammation associated with it are the cause of most human disease. This would suggest that free radicals are implicated in many disease states for example rheumatoid arthritis, asthma, stroke, or cancer. Therefore antioxidants are important to protect against oxidative damage, thus reducing the risk of inflammation. There are a number of antioxidants which play a protective role the body, such as ferritin, superoxide dismutase, transferrin, uric acid and glutathione reductase.
Ferritin
Ferritin is responsible for storing iron and releasing it when required. Ordinarily, ferritin is found inside blood cells with only a small amount circulating in the blood. Ferritin is clinically significant at both high and low levels. Low levels of ferritin can highlight an iron deficiency which causes anaemia. Whereas elevated levels of ferritin can be a result of conditions such as rheumatoid arthritis, haemochromatosis, liver disease, metabolic syndrome, type 2 diabetes and renal failure.2 As ferritin is an acute phase reactant, levels will be elevated in any inflammatory state within the body.3
Transferrin
Transferrin is a protein that is responsible for binding and transporting iron in the blood. Transferrin acts as a preventative antioxidant as it binds with free iron, removing it from the bloodstream. This is a critical function, as free iron can stimulate the production of harmful free radicals. As transferrin is a negative acute phase protein, lower levels are associated with inflammatory conditions.7
Superoxide Dismutase
Superoxide is a by-product of oxygen metabolism and is one of the most damaging free radicals in the body as it can cause cell damage. Superoxide Dismutase (SOD) is an enzyme which catalyses the breakdown of superoxide into a less damaging oxygen or hydrogen peroxide. Therefore SOD preforms a vital defensive function to reduce oxidative stress.4 Extensive research exists which links oxidative stress to chronic inflammation, which can be a contributing factor to diabetes, arthritis, cardiovascular disease and cancer.5 Therefore if levels of superoxide dismutase are low, patients are at risk inflammation, for example, SOD levels are significantly less in rheumatoid arthritis patients.6
Glutathione Reductase
Glutathione reductase is found in red blood cells and plays a key role in maintaining cell function and preventing oxidative stress in human cells. Reduced levels of glutathione reductase can contribute to the prevalence of inflammatory states, suggesting that adequate levels of glutathione reductase are essential for optimal function of the immune system. 7, 8
Uric Acid
Uric acid is a waste product produced when the body breaks down chemical compounds called purines. It is a scavenging antioxidant that acts by inactivating free radicals. Elevated levels of uric acid is commonly associated with gout, a type of arthritis which is caused when crystals of sodium urate form inside joints causing rapid and painful inflammation.9 Other research has indicated that elevated levels of uric acid is associated with increased risk of cardiovascular disease.
Total Antioxidant Status (TAS)
TAS is a measurement of antioxidant function rather than quantity and considers the cumulative effect of all antioxidants present. The antioxidant defence system has many components, and a deficiency in any of these components can cause a reduction in the overall antioxidant status of an individual.10 Reduction in total antioxidant status has been implicated in several disease states including cancer, CVD, Arthritis and Alzheimer’s disease.
As demonstrated above, different types of antioxidants can help reduce different types of inflammation. Antioxidant tests can be requested from any doctor, who may also review dietary intake, investigate any symptoms and advise if testing is required. If antioxidant levels are found to be inadequate, improving them can be easily done through dietary changes, and can help reduce a body’s overall inflammation.
For health professionals
Randox Laboratories offer a range of diagnostic reagents for antioxidant testing to assist in the diagnosis of inflammatory diseases. Randox offer a complete diagnostic package with applications for a range of biochemistry analysers and a selection of kit sizes, controls and calibrators available. Available tests include: Ferritin, Transferrin, Superoxide Dismutase (Ransod), Glutathione Reductase, Uric Acid, and Total Antioxidant Status (TAS).
References:
- Nordqvist, C., Inflammation: Causes, Symptoms and Treatment. Medical News Today, 2015, https://goo.gl/rT4WS9 (accessed 16 January 2017)
- Koperdanova, M., Interpreting raised serum ferritin levels, British Medical Journal, 2015, https://doi.org/10.1136/bmj.h3692 (accessed 2 February 2017)
- Nall, R. Ferritin Level Blood Test, Health Line, 2015, https://goo.gl/XGcW9P (accessed 2 February 2017)
- Yasui, K. and Baba, A., Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflammation Research. Vol.55, No.9, pp.359-363, 2006, 1007/s00011-006-5195-y (accessed 2 February 2017)
- Reuter, S., Gupta, S.C., Chaturvedi, M.M., Aggarwal, B.B., Oxidative stress, inflammation and cancer: How are they linked? Free Radic Biol Med. 2010, 1; 49(11):1603-1616 https://goo.gl/Uez3JZ (accessed 2 February 2017)
- Bae SC, Kim SJ, Sung MK., Inadequate antioxidant nutrient intake and altered plasma antioxidant status of rheumatoid arthritis patients. J Am Coll Nutr. 2003 Aug;22(4):311-5
- Reynolds, B., Glutathione for inflammatory respsonse, FX Medicine, 2015, Available from: https://goo.gl/2YAv5l (accessed 3 February 2017)
- Morris, G., Anderson, G., Dean, O. et al., The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol 2014;50(3):1059-1084, Available from: https://goo.gl/PDSgwv (accessed 3 February 2017)
- Malaghan Institute, Uric acid – a new look at an old marker of inflammation, Malaghan Institute of Medical Research, 2013, Available from: https://goo.gl/P6NfXP
- Li, Y., Browne, R.W., Bonner, M.R., Deng, F., Tian, L., Mu, L., Positive Relationship between Total Antioxidant Status and Chemokines Observed in Adults. Oxid Med Cell Longev. 2014, Available from: https://goo.gl/rmj5MB (accessed 9 February 2017)

Inflammatory Biomarker Series: Rheumatoid Factor
What are inflammatory biomarkers?
The purpose of measuring an inflammatory biomarker is to detect inflammation, which can assist clinicians in the identification of a particular disease or provide a marker of treatment response. Inflammation, either chronic or acute, is the body’s immune response to protect against harmful stimuli such as damaged cells, irritants or pathogens.1 When inflammation occurs in the body, extra protein is released from the site of inflammation and circulates in the bloodstream.2 It is these proteins, or antibodies, which clinicians are testing for in the blood as they can indicate if inflammation is present.Like many inflammatory biomarkers, such as rheumatoid factor (RF), C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR), further tests will be required as testing for these tests alone does not provide a clearly defined diagnosis. However inflammatory biomarker tests can provide clinicians with a good indication of what may be wrong with a patient, which is why they are commonly tested for in a clinical setting.
What is Rheumatoid Factor?
Rheumatoid factor (RF) is an autoantibody which can target and damage healthy body tissue and in turn cause inflammatory symptoms.3 It is uncommon for this antibody to be present in healthy individuals, which is why it is a beneficial test to aid the diagnostic process. In particular, rheumatoid factor can be used as an inflammatory biomarker to assist in the diagnosis of rheumatoid arthritis (RA). However the rheumatoid factor antibody can also be present in healthy individuals or patients with systemic lupus erythematosus, liver cirrhosis, Sjögren’s Syndrome, Hepatitis and other conditions.4 If a test detects rheumatoid factor levels above 14 IU/ml, this is considered abnormally high.3
What is Rheumatoid Arthritis?
Rheumatoid arthritis is an autoimmune disease which attacks the lining tissue of joints, resulting in chronic inflammation. This disease commonly affects the hands, feet and wrists, with symptoms causing pain, fatigue and loss of bodily function and over time may even lead to multiple organ damage.5 Although diagnosis of rheumatoid arthritis requires a physical examination, testing for rheumatoid factor can be beneficial to assist in the diagnosis of this disease. Other blood tests that can be used to detect biomarkers associated with rheumatoid arthritis include C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), IgA, IgG, IgM and anti-cyclic citrullinated peptide (anti-CCP).
For health professionals
Randox Laboratories offer a leading portfolio of diagnostic reagents which includes a test for rheumatoid factor, with applications available for a range of biochemistry analysers. With a measuring range of 6.72 – 104 lU/ml, this assay can comfortably detect levels outside the normal range. Randox offer a complete diagnostic package for the screening of rheumatoid factor with a range of kit sizes, controls and calibrators available. Other inflammatory biomarker tests available from Randox include CRP, High Sensitivity CRP, Full Range CRP, IgA, IgG and IgM.
References:
1. Nordqvist, C. Inflammation: Causes, Symptoms and Treatment. Medical News Today, https://goo.gl/rT4WS9 (accessed 16 January 2017)
2. Harding, M., Blood Tests to Detect Inflammation, Patient, 2015, https://goo.gl/F4OGrz, (accessed 16 January 2017)
3. Shiel, W. C., Rheumatoid Factor (RF), MedicineNet, 2016, https://goo.gl/XPA69u 2016 (accessed 16 January 2017)
4. Rheumatoid Arthritis Organisation, Rheumatoid Factor Test, Rheumatoid Arthritis Organisation, 2016, https://goo.gl/JujE5a
5. Gibofsky, A. Overview of Epidemiology, Pathophysiology and Diagnosis of Rheumatoid Arthritis. The American Journal of Managed Care. Vol.18, No.13. p.295-302, 2012

The Benefits of Peer Group Data to your Troubleshooting Process
Drive for more accurate results in your laboratory
We’ve all been there, you’re in the middle of a run of patient tests when you are alerted to an out of control event, such as your analyser is reporting QC results 25% low to target. What do you do? In reality, we all know that the problem is unlikely to correct itself, especially if it’s a calibration or analyser issue. Human error is a potential factor, however all possible causes must be eliminated to proceed with patient testing.
What’s the solution?
ISO 15189:2012 recommends that a laboratory should “have a procedure to prevent patient results in the event of a quality control failure”. Implementing an interlaboratory data management program which features peer group reporting can help you meet this requirement and monitor the results you are producing. Such programs can help detect errors in the analytical phase of patient testing, through the automatic application of pre-programmed QC rules, thus alerting staff to failed results.
Why must Peer Groups be a feature?
A peer group is defined as a “Community in which most or all members have roughly the same characteristics…” (Businessdictionary.com, accessed 2017). In this instance the characteristics could refer to the; instrument, test method or QC material in use. As such peer group programmes could help you detect errors in your laboratory by comparing your results to those who are employing a similar method, instrument and QC to what you are using, i.e. comparing apples for apples. Therefore it is essential that the peer group data you require is available in real-time, to ensure you are accessing the most up-to-date data when reviewing your patient test results.
Scenario
Take the example from the introduction. You’re in the middle of a run of patient tests when you are alerted to an out of control event, such as your analyser is reporting QC results 25% low to target. As part of your troubleshooting procedures, you are able to compare your results to the results of your peer group and note that this is an isolated incident. Consequently, you have eliminated a widespread problem with the QC, reagent or calibrator and narrowed down the root cause to one of the components in your test system. Thus saving you time in the troubleshooting process.
Benefits of Peer Group Comparison
There are a number of benefits to employing peer group comparison in your laboratory. Peer group data comparisons facilitate faster troubleshooting, helping you identify whether the problem you are seeing is unique to your laboratory, or if other laboratories are reporting the same issue. If other laboratories are reporting the same issue it is possible to conclude that there is a widespread problem with either the QC, reagent or calibrator. On the other hand, if it is not occurring within your peer group you will have to investigate further, reviewing your QC processes. As a result, you could resolve issues much quicker by eliminating either a supplier or laboratory issue. Furthermore, you can also eliminate the need for unnecessary repeat tests or instrument maintenance, saving both valuable time and money.
Other characteristics you should look out for
Whilst peer group comparison is a useful feature there are a number of other features you should consider when selecting the right interlaboratory data management program for you. These include;
- Automatic calculation of Measurement Uncertainty, Total Error and Sigma Metrics
- Multiple laboratory management on a single platform
- Accessing data anytime, anywhere via PC, laptop or tablet via a web-based platform
- All data charts you may require to assess whether any bias or imprecision issues are present
- Ability to combine data for multiple QC lots, analytes and instruments on a single Levey-Jennings or Histogram chart
- Automated data import via a direct connection to your LIMS
What can Randox offer?
At Randox we are passionate about quality control and believe in producing high-quality material that can streamline procedures for laboratories of all sizes and budgets through our Randox Quality Control brand. Acusera 24.7 Live Online is just one aspect of our extensive laboratory portfolio that has been designed to help you produce results you can trust. With Acusera 24.7 Live Online you can drive for more accurate results by monitoring and interpreting QC data online, anytime, anywhere. With access to an impressive range of features, including the automatic calculation of Measurement Uncertainty, Total Error and Sigma Metrics, Acusera 24.7 will ensure analytical quality.
Acusera 24•7 Live Online – Speeding up the Review of QC Data
Reviewing QC data can be an extremely time consuming and costly process. With manual statistical calculation laboratories risk missing or ignoring significant trends in QC data which could potentially put patients at risk. So how does a laboratory combat this? Simple; participate in an interlaboratory data management program that provides a quick, effective, accurate and detailed analysis of QC results. The answer to this program is Acusera 24•7 Live Online.
Acusera 24•7 Live Online
With the launch of Acusera 24•7 Live Online version 2.0, QC data review is now faster and simpler than ever before. Our program aims to save the laboratory precious time and money by instantly flagging any QC failures, ultimately ensuring accurate test system performance.
Designed to complement and be used primarily with our Acusera range of true third party controls, Acusera 24•7 Live Online has two primary functions; 1) management and interpretation of IQC data and 2) rapid and effective troubleshooting of QC failures via access to instantly updated worldwide peer group statistics.
These two functions have one common goal – being an effective tool for evaluating laboratory performance. With the launch of version 2.0 the software boasts even more functionality than before, ensuring any laboratory employing Randox Quality Control coupled with Acusera 24•7 Live Online will see benefits from the get-go.
Why should you use Acusera 24•7 Live Online?
Using Acusera 24•7 to help speed up the review process in your laboratory can reap dividends. The program has been designed for this specific reason and the features are geared towards helping the laboratory review, interpret, and analyse QC data quickly, effectively and accurately. One such example of this is the unique dashboard function which instantly flags any alerted or rejected results from the past 7 days, significantly reducing the time spent analysing reports and charts whilst simultaneously allowing any corrective action to be taken immediately with minimum disruption to the lab’s output.
Previously, peer group statistics would have been updated every 24 hours with Acusera 24•7 Live Online version 1.6, however, with the new release, peer data is about to get a unique upgrade. Gone are the days when you will have to wait 24 hours to get updated stats – Acusera 24•7 Live Online now has the ability to generate peer data live in real-time, thereby enhancing the laboratory’s troubleshooting capabilities and allowing labs to compare their data with others around the globe. What’s more there is no deadline for submission of results meaning labs can get a true reflection of performance at any time. Ultimately, laboratories will be able to easily identify if an issue is unique to them or a widespread issue amongst their peers. Such information will allow them establish a root cause quicker and spend less time troubleshooting.
The capacity to generate interactive charts and comprehensive reports automatically is a feature included in Acusera 24•7 that will aid quick review of QC data. Reports can be generated for a user-defined date range and provide a wealth of information. Reports include statistical analysis, statistical metrics, measurement uncertainty, exception and audit trail reports. Reports coupled with Levey-Jennings, Histogram and Performance Summary charts enable rapid and stress-free performance monitoring. The ability to add multiple instruments, QC lots and analytes to a single chart allows for comparative performance assessment and immediate identification of any trends.
We must not forget that Acusera 24•7 Live Online has already had a modernisation in the past few months. In November 2016 we announced the automatic calculation of Measurement of Uncertainty, Total Error and Sigma Metrics. These new features are also included in the version 2.0 launch of our Live Online program.
Our software is highly flexible with custom configurations of performance limits, multi-rules and target values designed to meet and exceed every laboratory’s needs.
With the ability to identify trends, system errors, minimise false rejections and bridge the gap between IQC and EQA, there really is no reason to look elsewhere for your analytical performance of QC.
For more information on Acusera 24•7 Live Online or our Acusera third party controls, click here.





