Advanced Statistics with Acusera 24.7
Advanced Statistics with Acusera 24.7
The only thing that sounds more terrifying than statistics, is advanced statistics. For many of us, the dread associated with having to carry out complex calculations can be too much to bear. For others, statistics are not just a set of numbers; they’re a captivating puzzle waiting to be solved. The allure of dissecting intricate patterns, unravelling hidden relationships, and drawing meaningful conclusions makes these statistical enthusiasts embrace the challenges of advanced statistics with excitement rather than apprehension.
No matter which camp you’re in, we bet you’re going to love the advanced statistics features included in Acusera 24.7. From Uncertainty of Measurement to Sigma Metrics, we’ve got you covered. Let’s explore these features and how we can make your statistical analysis easier than ever before.
Measurement Uncertainty
If you’re involved in laboratory quality control, you’ll have heard all about measurement uncertainty (MU). To some it’s intuitive. To some it’s a labyrinth. MU is defined as a parameter associated with the result of a measurement that characterises the dispersion of values that could reasonably be attributed to the measured quantity. For example, if we say the pencil below measures 16cm ± 1cm, at the 95% confidence level we are really saying that we are 95% sure that the pencil measures between 15cm and 17cm.

In other words, the calculation of MU gives medical laboratories an estimate of the overall variability in the values they report. This is important for 3 reasons:
- It helps ensure the measured results are useful and not wildly inaccurate.
- It permits meaningful comparison of medical decision limits and previous results of the same kind in the same individual.
- It’s a regulatory requirement – ISO 15189:2022
All measurements involve some degree of inherent variability due to factors such as instrument limitations, environmental conditions, and biological variation. MU aims to quantify the doubt or range of possible values around the measurement result, helping to provide an understanding of the reliability and limitations of measurements. To complete this task comprehensively, the entire measurement process must be examined and should consider components such as systematic errors, random errors and uncertainties related to calibration, equipment, and the environment.
ISO 15189:2022 states:

So, if you are seeking ISO15189 accreditation, there’s no avoiding MU and advanced statistics. Lucky for you, Acusera 24.7 can calculate MU and provide you with a report which you can export to Excel or PDF for auditing or archiving.
By liberating you from the need to manually calculate MU for all your assays and control levels, Acusera 24.7 streamlines the statistical analysis process, freeing you up to complete your other essential duties. It also helps reduce the chance of errors in the calculation; after all, no matter how talented you are at mathematics, we all make mistakes. The real-time nature of this kind of monitoring means you don’t have to recalculate every time you get more data – simply press the refresh button and you’ll automatically get a new MU report.
By incorporating automated tools to calculate MU, you gain the ability to proactively pinpoint and rectify potential error sources, mitigating the risk of inaccurate measurements and the repercussions that may follow.

For more information on MU and how it’s calculated, see our education guide – How to Measure Uncertainty.
Sigma Metrics
The Sigma model was originally developed for the manufacturing industry as a method of process improvement focusing on minimising errors in process outputs. It has since been adopted by the medical laboratory to improve result reporting.
This model calculates the number of standard deviations or ‘Sigmas’ that fit within the quality specifications of the process – as the sources of error or variation are removed, the standard deviation becomes smaller, and the sigma score increases – 6 being the target. A 6 Sigma process can be expected to produce 3.4 defects, or false results, per million.

Using your predetermined performance limits, including biological variation (standard), RiliBÄK and CLIA, as the total allowable error (TEa), Acusera 24.7 can calculate a Sigma Score for a particular assay, method, or instrument, saving you the hassle of calculating this manually – freeing you up to investigate the sources of error and make improvements to your process.
This is displayed in our Statistical Metrics report along with Count, Bias%, and CV for your chosen range, your cumulative results and those from other Acusera 24.7 users from around the world to provide straightforward and comprehensive statistical analysis and peer group comparison.

Once you’ve found out your Sigma Score for an assay, you can use this to determine your QC frequency and the multi-rules you should apply to your QC. The higher your Sigma Score, the less multi-rules you need to apply to your analysis and the less often you need to run QC for that assay. The table below shows the multi-rules and QC frequencies associated with each Sigma Score.

Acusera 24.7 includes multi-rule capabilities that can be utilised to monitor your QC data and index it as accepted, rejected, or trigger an alert, depending on the pre-defined multi-rules against which you want to check your data. These features enable the identification of nonconformities and reduce the need for laborious manual statistical analysis while enhancing the accuracy and precision of the laboratory. To read more about the multi-rule features of Acusera 24.7, take a look at our educational guide – Understanding QC Multi-rules.

Now that we’ve found which of our assays are underperforming, we can begin to take corrective action. The Sigma Score is affected by bias and imprecision of laboratory results, therefore improving these values will increase the Sigma Score. Some of the steps a laboratory can take are:
- Improved staff training
- Instrument maintenance
- Frequent calibration
- Strict adherence to SOPs when preparing controls and calibrators.
If you are still in the dark ages, carrying out your statistical calculations and analysis manually, reach out to us today to learn more about the time and expense we can help you save. Every day, more people are discovering the power of Acusera 24.7 and the benefits it has in their laboratory.
The updates to ISO151589:2022 are based around increasing patient safety and reducing erroneous results, making advanced statistics essential. Assessors get excited when they see Acusera 24.7 in the lab because they know quitting time is that bit closer. Allow us to help you achieve your accreditation and provide the best possible patient care. With complete onboarding assistance and first-class customer support, you’ll always be ready to get to the bottom of any problems you might face. Get in touch today at marketing@randox.com
World Diabetes Day

World Diabetes Day!
14th November
Diabetes is a life-long condition that affects millions of people worldwide. It causes blood sugar levels to become abnormally high, which poses a significant danger to your body. Although it’s incurable, it can be monitored and controlled.
Randox Laboratories provide a range of diabetes related diagnostic reagents, that cover many areas from disease recognition and diagnosis to monitoring symptoms and complications associated with the condition.

The three main types of Diabetes are Type 1, Type 2 and Gestational Diabetes.
Type 1 diabetes starts in early childhood, and is caused by a deficiency in the production of insulin by the pancreas. It can be both inherited and acquired. Daily monitoring is required, along with the administration of insulin. Type 1 is when your body can’t naturally produce the hormone insulin, vital for the transportation of glucose in the blood to the cells to energize and fuel our bodies. Your body attacks cells within the pancreas, meaning the body is unable to produce insulin. Without insulin there is no glucose getting into bodily cells, so it builds up in your blood stream causing high blood sugar levels.
Type 2 manifests later in life, it’s when the body produces insulin but does not use it effectively. Can be referred to as insulin resistance. It is more common than Type 1 and usually can be the cause of lack of exercise and excess body fat. However, regardless of weight it can occur in any individual. Type 2 diabetes can be regulated by your diet. Without treatment or action, diabetes can cause many serious problems like damaging the eyes, feet and heart. Some symptoms may include urinating more than usual, feeling lethargic, weight loss, blurred vision and wounds or cuts taking longer to heal.
Gestational diabetes is the development of the condition during pregnancy. It is the result of the body being unable to produce enough insulin to meet the extra needs of the baby and mother. It can cause serious problems if not controlled, to reduce the risks Gestational diabetes is consistently monitored.
Randox Laboratories provide an extensive range of reagents for the accurate testing of diabetes. One of the reagents is Fructosamine in which ‘testing has been identified as being the best for patient care’.
Fructosamine & HbA1c
Fructosamine is a mid-term indicator of diabetic control, as it can provide information on a person’s average blood glucose levels over the previous 14-21 days. Because of it’s shorter time span, it’s often used to evaluate the effectiveness of medication changes and to monitor the treatment of gestational diabetes.
Fructosamine is also particularly useful in situations where HbA1c cannot be reliably measured e.g. haemolytic anaemia, thalassemia or with genetic hemoglobin variants.

HbA1c for example – gives us an indication of what an individual’s average blood sugar level has been over recent weeks/months. This is significant for those who suffer from diabetes because the higher the levels of HbA1c, the higher the chance of an individual suffering from further diabetes related issues.
The latex enhanced immunoturbidimetric method which the RX series utilises makes, the test simple and quick to perform. The removal of the pre-dilution step removes the risk of human error compromising your results. Certified by the National Glycohemoglobin Standardization Program (NGSP), the RX daytona+, RX imola & RX modena are all capable of utilising direct on-board HbA1c which can revolutionise your diabetes testing capabilities.
D-3-Hydroxybutyrate (Ranbut)

D-3-Hydroxybutyrate (Ranbut) is another reagent Randox provides. D-3-Hydroxybutyrate is the most abundant of the three main ketones produced in the body, accounting for 75% of total ketones in the body. Due to the higher levels of D-3-Hydroxybutyrate, it is the more sensitive marker for the diagnosis of ketosis.
Ketosis is a metabolic process, occurring when the body switches from glucose to predominantly fat metabolism for energy production, this happens when carbohydrate availability reaches low levels. The metabolism of fatty acids in the liver, results in the production of chemical by-products known as ketone bodies or ketones. Ketosis occurs when the body produces more ketones than the liver can process.
High levels of ketones present in the body can be dangerous leading to Diabetic Ketoacidosis, which being left untreated can cause damage to vital organs and in some instances may lead to a coma or death.
Benefits of Ranbut include;
• Superior methodology when compared to other commercially available ketone detection tests. For example, the nitroprusside method used in semi-quantitative dipstick tests only detects acetone and acetoacetate. D-3 hydroxybutyrate is the most abundant ketone produced during ketosis the measurement of this analyte is more sensitive and specific.
• Exceptional correlation coefficient of r=0.9954 when compared against other commercially available methods.
• Excellent precision of <3.5% CV.
• Calibrator and controls available offering a complete testing package.
• Applications available detailing instrument-specific settings for the convenient use of the Randox D-3- Hydroxybutyrate (Ranbut) assay on a wide range of clinical chemistry analysers.
• New liquid stable Ranbut assays available.
Non-Esterified Fatty Acids (NEFA)
Non-Esterified Fatty Acids is another reagent Randox provides, which are important metabolites stored in adipose tissue. The dominant source of NEFA is abdominal subcutaneous fat. Cross-sectional studies have consistently documented that circulating NEFA levels are proportional to body fat storage and demonstrated positive correlations between fasting NEFA levels and obesity, insulin resistance and glucose tolerance. It too is an accurate marker regarding diabetes.
Non-Esterified Fatty Acids concentrations are strongly associated with insulin resistance. In the fasting state, the resistance of adipose tissue to the antilipolytic effect of insulin causes the extensive release of NEFA into circulation. Consequently, elevated NEFA levels exacerbate insulin resistance through diminishing insulin- stimulated glucose intake into the skeletal muscle, directly affecting insulin signalling.
Benefits of NEFA include;
• Exceptional correlation coefficient of r=0.98 when compared against other commercially available methods.
• Excellent precision of <5% CV.
• Extensive measuring range of 0.072- 2.24mmol/l for the comfortable detection of clinically important results.
• Calibrator and controls available offering a complete testing package.
• Applications available detailing instrument-specific settings for the convenient use of the Randox NEFA assay on a wide range of clinical chemistry analysers.

Randox’s Reagents available for diagnosis and monitoring of diabetes.
Fructosamine
Glucose
HbA1c
Albumin
Creatinine
Cystatin C
D-3-Hydroxybutyrate
Microalbumin
Non-Esterified Fatty Acids
‘The Randox enzymatic method offers, improved specificity and reliability compared to the conventional NBT-based methods, as the enzymatic method does not suffer from non-specific interferences, unlike the existing methods which can also be time-consuming and difficult to automate. The Randox dedicated Fructosamine calibrator and controls are assigned relative to human serum glycated with 14-C glucose, directly reflecting the nature of the patient sample. Randox provides testing and reagents that are reliable and accurate.’
References
Anon (2023) Types of diabetes, Diabetes UK. Available at: https://www.diabetes.org.uk/diabetes-the-basics/types-of-diabetes (Accessed: 19 September 2023).
NHS (2023) Symptoms of Diabetes, NHS Choices. Available at: https://www.nhs.uk/conditions/type-2-diabetes/symptoms/ (Accessed: 19 September 2023).
Randox (2023) Diabetes Reagents: Biochemistry: Reagents, Randox Laboratories. Available at: https://www.randox.com/diabetes-reagents/ (Accessed: 19 September 2023).
Randox (2023b) Fructosamine: Reagents: Biochemistry, Randox Laboratories. Available at: https://www.randox.com/fructosamine/ (Accessed: 20 September 2023).
David Davis calls for blanket testing to save NHS £3bn a year

Ministers are being urged to introduce mass medical testing to cut NHS costs by billions and save thousands of lives.
Former Brexit Secretary David Davis calls for mass medical testing across the whole UK population by companies which he believes will alert the NHS to potential health risks for individuals and allow for lifestyle changes and treatment before problems become serious.
Mr. Davis said, “I bring it back to the reality of individuals. If we delay diagnosis, we delay treatment – we sentence people to death. It’s as harsh as that. So one of the things I would like to see us do is dramatically increase the amount of diagnostic capacity we have.”
“My view is that actually we should break clear of the ideology. We should look to increase dramatically the amount of scans and diagnostic procedures we can create. And when I say dramatically, I mean a multiple of what we currently do and we should use the private sector to do it.
“I know it causes a bridling and a backing off but I the only way we can do it fast enough is to do that. And that will save I think about £3 billion, get the waiting lists down by millions of people but most importantly of all will save thousands of lives.”
Mr. Davis was reacting to a paper drawn up by Northern Ireland medical testing company Randox, one of the private health providers who developed Covid testing during lockdown. The paper has been drawn up by scientists at Randox, scaling up its testing capacity from 300 tests a day to 120,000 a day in less than 12 months. Overall, the firm conducted nearly 27 million tests during the pandemic.
Dr. Peter Fitzgerald, the founder of Randox, said that with the NHS waiting lists not far short of eight million people and with budgets under intense pressure, the time had come for a new partnership between the public and private sectors.
Ministers should start by convening a summit of private diagnostic firms and their NHS counterparts and investigate the potential of the enormous advances in testing technologies developed in recent years. By harnessing the startling progress made by scientists they could revolutionize standards of health care while slashing waiting lists and achieving far greater value for money.
Dr. Fitzgerald added, “Policy-makers need to appreciate the vast potential of the latest diagnostic testing technologies. They can deliver a step-change in the quality of people’s lives. By outsourcing much testing to the private sector – under a rigorous independent tendering process – the NHS can be freed up to get on with its prime job of treating the sick.”
Under the Randox plan, the public would be invited to visit a private diagnostic clinic every year for a check-up. Results would be monitored in house by scientists who would advise people on next steps. Results would be routinely passed onto their GPs, though in many cases no further action would be needed.
GPs would ultimately decide on medical interventions and possible referral to NHS hospital services. A priority group for such tests would be the 7.7 million on NHS waiting lists. They would be assessed to see if their condition had worsened and whether urgent action was required.
High-tech comprehensive testing of the population would also reduce if not eliminate the many false positives arising from much of the diagnostic services available today.
Taken from Daily Express article by David Maddox, Political Editor.
Randox welcomed the Queen’s University staff Leadership Team to their Antrim based Randox Science Park in Antrim.
Randox were delighted to welcome the Queen’s University Belfast Leadership Team to their Antrim based headquarters, Randox Science Park on Tuesday, October 4th.
President and Vice Chancellor, Sir Ian Greer of Queen’s University was joined by Deputy Vice-Chancellor Professor Stuart Elborn, Head of Careers, Employability and Skills Mr Trevor Johnston, Head of Business Alliance Mr Dermot Leonard, Business Engagement Manager Mrs Joanne Mallon, Executive Director of the Global Innovation Institute Dr. David Quinn, Lead of Queen’s sustainable energy research Professor David Rooney, Dean of Research in Medicine, Health and Life Sciences Professor David Rooney, Dean of Research in Medicine, Health and, Life Sciences Professor Chris Scott, and Dean of Impact and Innovation in Medicine, Health, and Life Sciences.
The team received a presentation on Randox’s capabilities which stimulated multiple discussions in relation to research, improvements in healthcare provision, skills and the exciting future of Life and Health Sciences in Northern Ireland.
The purpose-built facilities at the site, covering research and development, engineering, manufacturing and accredited laboratories provides an unparalleled depth of diagnostic capability within a single site.
Randox employ over 2,200 staff, including 800 research scientists and engineers – all focused on improving life science diagnostic capabilities globally.
More than 5% of the world’s population (over 400 million people) receive medical diagnosis using Randox products each year. Randox have major facilities in the UK, Ireland, India, and the United States, supported by global distribution and supply networks.

Secretary of State visit to Randox Science Park
The Rt Hon Chris Heaton-Harris, Secretary of State for Northern Ireland paid a visit to Randox Science Park on Thursday, October 19th, to discuss Randox capabilities and undertake a tour of the facilities at the Antrim site.
As leading diagnostic company from the UK & Ireland, Randox have spent over forty years improving healthcare, with a focus on the provision of timely and accurate testing both to improve clinical diagnosis and promote preventative healthcare.
The purpose-built facilities at the site, covering research and development, engineering, manufacturing and accredited laboratories provides an unparalleled depth of diagnostic capability with a single site.
More than 5% of the world’s population (over 400 million people) receive medical diagnosis using Randox products each year.
Randox’s proprietary Biochip Technology is the result of a £350 million investment, allowing many tests to be run simultaneously, greatly improving the diagnostic power available to clinicians. This innovative technology allowed the provision of advanced health profiling to support both early diagnosis and the transition to preventative healthcare.
Randox Science Park is a central hub of Randox’s life science manufacturing, engineering and research and development. Randox employ over 2,200 staff, including 800 research scientists and
engineers – all focused on improving life science diagnostic capabilities globally.
Randox Science Park is one of four key manufacturing and development sites, with others located in Dungloe, County Donegal; Bangalore, India; and the Greater Washington DC area, USA. Across the UK & Ireland there is also a growing network of Randox Health Clinics.

Look after your gut and it will look after you – Goodwood Health Summit 2023

At long last the public is cottoning on to the simple but important notion of preventative health – the idea that you don’t go to the doctor after falling ill – you go before so that potential future illnesses can be identified in advance and action taken immediately.
Randox, a leader in the field of diagnostic medicine, is in the forefront of this profound change in health care – one that opens up the possibility of delivering enormous benefits to individuals and society at large.
For these reasons, we were delighted to lend our support to the recent launch of the Goodwood Gut Summit hosted by the Goodwood Estate. The summit theme was on gut microbiomes, which play a key role in promoting the smooth daily operations of our body. Broadcast online, the summit aimed to respond to the urgent need for widespread education and communication about rapid progress in dietary health.
The summit came after a stark warning contained in two landmark studies into the effects of ultra-processed foods on our diet and its effect on our microbiomes.
This newly published research concluded that eating ultra-processed foods – such as ready meals, fizzy juice, cereals, and fast food – drastically increases our risk of serious health issues, such as high blood pressure and diabetes. It can also raise the risk of heart attacks and strokes. BBC journalist Justin Webb led the conversation with a world-class line-up of speakers, including, Dr Chris van Tulleken, Jessie Inchauspé, Dr James Kinross, Professor Pekka Puska and Professor Edward Bullmore.
Topics covered included inflammation, mental health and the microbiome , insulin, obesity, ultra-processed foods , the growing cost of poor nutrition, and the need to drive fundamental shifts in our food systems in order to move to a healthier future for all.
There was a discussion on using the many curbs on the promotion and sale of tobacco as a model for the food industry. Tighter regulation of food manufacturers and their marketing strategies could be the way forward here. As authorities in their respective fields, the speakers shared their knowledge and vision on these important topics, as well as considered new solutions to personal and societal health challenges, helping the formulation of some key achievable goals.
The partnership with the summit is underpinned by two Randox Laboratory divisions.
Randox Food Diagnostics is dedicated to improving the global food security chain. It provides the global food market with screening solutions for antimicrobials, toxins, growth-promoting hormones and veterinary drugs in animals and animal produce, as well as testing meat, milk, honey, grapes, seafood and feed products.
Food product testing is essential to ensure that what we consume is safe from physical, chemical, and biological hazards. It tells people precisely what they are eating and so helps them make informed choices and makes sure that goods on the supermarket shelves comply with safety standards.
Randox Health, the consumer-facing side of Randox Laboratories, is primarily focused on accessible, preventative health testing and offers full body health checks that identify early signs of disease before symptoms occur.
What is gut microbiome?
Picture a bustling city on a weekday morning, the pavements flooded with people rushing to get to work or to appointments. Now imagine this at a microscopic level and you have an idea of what the microbiome looks like inside our bodies, consisting of trillions of microorganisms (also called microbiota or microbes) of thousands of different species.
These include not only bacteria but fungi, parasites, and viruses. In a healthy person, these “bugs” coexist peacefully, with the largest numbers found in the small and large intestines but also throughout the body. The microbiome is even labeled a supporting organ because it plays so many key roles in promoting the smooth daily operations of the human body.
A person is first exposed to microorganisms as an infant, during delivery in the birth canal and through the mother’s breast milk. Later on, environmental exposures and diet can change our microbiomes to be either beneficial to health or to place one at greater risk for disease. Read more about it here(https://www.hsph.harvard.edu/nutritionsource/microbiome/)
More info on Randox Food Diagnostics: Randox food diagnostics-randoxfood.com
To book a health test please follow the link below; Randox Health-randoxhealth.com
Book your stay at the Goodwood Gut Health programme, that includes a Randox panel of testing; www.goodwood.com/visit-eat-stay/health-wellbeing/wellness-retreats/gut-health-programme/
Industry And Academic Partnership in Developing Type 1 Diabetes Genetic Risk Biochip
 The development of a diagnostic biochip to assess the genetic risk of individuals developing type 1 Diabetes, is the result of a successful partnership between leading diagnostics company, Randox and the University of Exeter.
The development of a diagnostic biochip to assess the genetic risk of individuals developing type 1 Diabetes, is the result of a successful partnership between leading diagnostics company, Randox and the University of Exeter.
The following case study has been prepared on the dynamic biochip’s development. With significant potential for further advancements in research and diagnostics, this active collaboration highlights how industry and academia can work together to accelerate healthcare innovation.
As a professor in Diabetes at the University of Exeter Medical School, Dr. Richard Oram specializes in the study of the biology of beta cell loss in type 1 diabetes and the clinical impact of persistent beta cell function. Working alongside Professor Michael Wheedon and Professor Andrew Hattersley, in 2014, Dr. Oram developed a method of assessing genetic risk as a single number – a genetic risk score (GRS) – that can be used to help classify what type of diabetes people have and predict future typ1 1 diabetes but to deliver a clinical test, the research would need a collaborative partnership with a global innovator in healthcare diagnostics.
Unlocking the potential of a type 1 diabetes GRS
Dr. Oram’s early research on type 1 Diabetes included studying people with varying levels of beta cell destruction and the study of extreme early-onset type 1 diabetes diagnosed in infants under a year old. One key question was whether aggregating data for someone’s genetic risk for type 1 diabetes could be turned into a single number – a genetic risk score and could be used to help understand the disease process or even correctly identify the type of diabetes someone had.
In parallel, it’s become increasingly apparent that there is a significant issue of incorrect classification of type 1 diabetes, affecting treatment and complications risk. Dr. Oram asked that if a ‘person’ sits in the overlap of whether they might have type 1 or type 2 diabetes, can their genes be used to help understand the disease process or even correctly identify the type of diabetes someone had.
Revolutionizing diagnosis with the type 1 diabetes GRS diagnostic tool
With the common confusion and misdiagnosis of type 1 or type 2 diabetes, it’s estimated that up to half of people with diabetes receive the wrong treatment. This information was a good indicator that a diagnostic test would be a simple method of correct diagnosis. But alongside accurate type 1 and type 2 diabetes diagnosis, the GRS research and classification model can also help:
● Identification diagnostics to understand which people with diabetes may have a genetic mutation causing it and need genome sequencing to make the diagnosis
● Predictive diagnostics to learn whether someone will develop diabetes in the future
All research showed that it was relatively easy to generate a GRS for Richard and the team’s studies and that it was clinically valuable. The next step was to translate the research into a user-friendly and affordable diagnostic test that can be widely adopted worldwide – and find a healthcare diagnostics company that could make it a reality.
Industry and academia partnerships to accelerate innovation
Together, both Randox and the University of Exeter highlight the continued importance of improving disease prediction and prevention, the collaboration showcases the power of interdisciplinary partnerships between industry and academia in advancing healthcare.
Over a four-year period, Randox developed a biochip that uses genetic markers and a robust algorithm to assess an individual’s genetic risk for type 1 diabetes accurately. Randox’s expertise in the development, manufacture, and regulatory approval of the biochip made it a reality all driven by discovery research and a clinical understanding from Dr. Oram.
With neither team being able to achieve the same results without the other, recognizing the strengths both sides can offer to accelerate healthcare innovation is the key to a successful industry/academia partnership.
As a first-generation type 1 diabetes biochip, Dr. Oram continues collaborative research with Randox to advance its potential. And to further the partnership, Randox has committed a research grant of over £2m to study genetic risk scores for other autoimmune diseases, including coeliac disease and multiple sclerosis.
For more information please visit: www.medicine.exeter.ac.uk/clinical-biomedical/business-engagement-innovation
Effortless Data Management: Acusera 24.7 Reports
You’ve carried out your daily maintenance and run your IQC. You’ve got your results and now it’s time to type them up into one of your expansive spreadsheets. Reports
You’ve probably got your spreadsheet set up to calculate the required parameters already, but what if there’s an error in the formula? Or what if you make an error when entering your data? Or worse, what if you try to open the spreadsheet only to find that the file is corrupted or lost? If your Excel file is there, someone else might already be editing it, meaning you must wait until they’re finished before you can make any changes.
Even if you face none of these obstacles the labour-intensive statistics needed for performance review and validation might just keep you up at night.
Well, with Acusera 24.7, these concerns are history.
Whether you make use of our automated or semi-automated data entry options, you can be sure that the data put into the system is exactly that returned by your instrument. If you use the manual data entry option, we can’t remove the human error element – but with our simple and intuitive interface, we trust you’ll be flawless anyway. What’s more, the cloud-based nature of our software also means you won’t lose the data by mistake and unique access for each user allows multiple people to be logged in at the same time.

So, what next?
Well, you can view this data on our dashboard for fast and easy access to your results but delve a little deeper into Acusera 24.7 and you can access comprehensive, easy-to-read, customisable, reports designed to speed up the review process.
These reports include statistical analysis, exception reports, peer group statistics, uncertainty of measurement and advanced statistical metrics. The latter two we’ll look at in a dedicated article. For the others, however, let’s dive in and see how you could benefit from our range of extensive reports.
Statistical Analysis Reports
The first report we will look at is the statistical analysis report. This report allows you to view your IQC data from a specified date range, and compare it to your cumulative data, that is, all the IQC data you’ve collected since you began using that lot, as well as the peer group data for the same lot all within one screen. If you are part of a chain of laboratories, you can compare this data with your laboratory group to see how your lab stacks up by using the World/Group toggle button.
This report provides you with the count, mean, SD, CV, SDI and CVI for a lot and can be organised by assay, as shown in the image below, instrument, or method, allowing you full freedom to customise this report to suit your needs. Don’t forget, like all our reports and charts, this data is fully exportable to PDF or Excel for filing or data review.
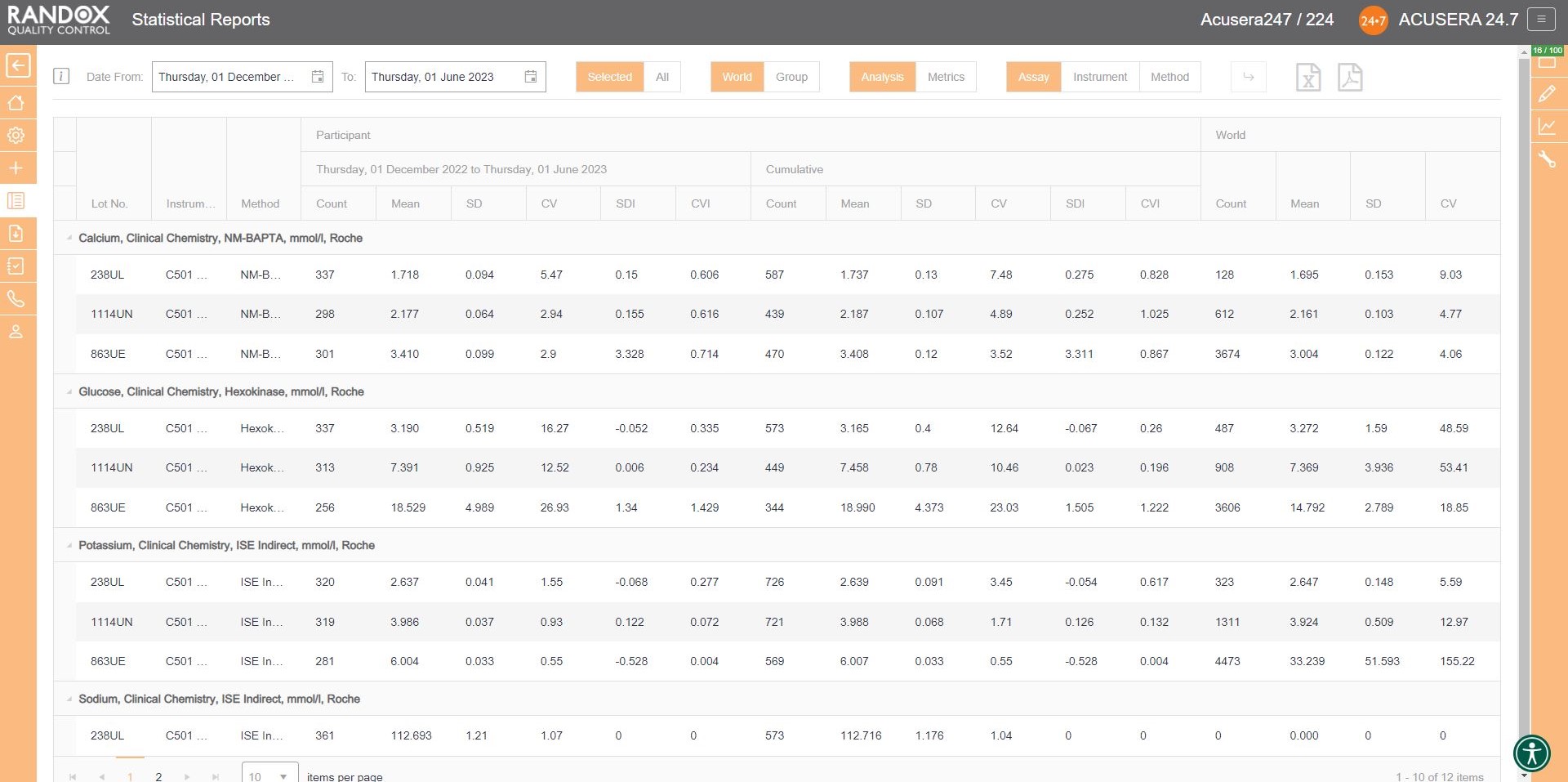
Handy, right? This report provides you with everything you need to carry out the validation and verification of new IQC lots, plus much more. We’ll look at this in more detail in an upcoming article.
Exception Reports
If you wish to determine your best and worst-performing tests, our exception report is perfect for you. This report is designed to quickly and easily identify assays with a high percentage of errors. The exception report provides an on-screen summary of the number of QC results for each individual assay and control lot that fall within the following categories: <2SD, 2-3SD and >3SD. This comprehensive performance review can be filtered: by clicking on the top of the ‘>3DSI’ column, this report will display assays in descending order with your worst-performing assays at the top, as shown below.
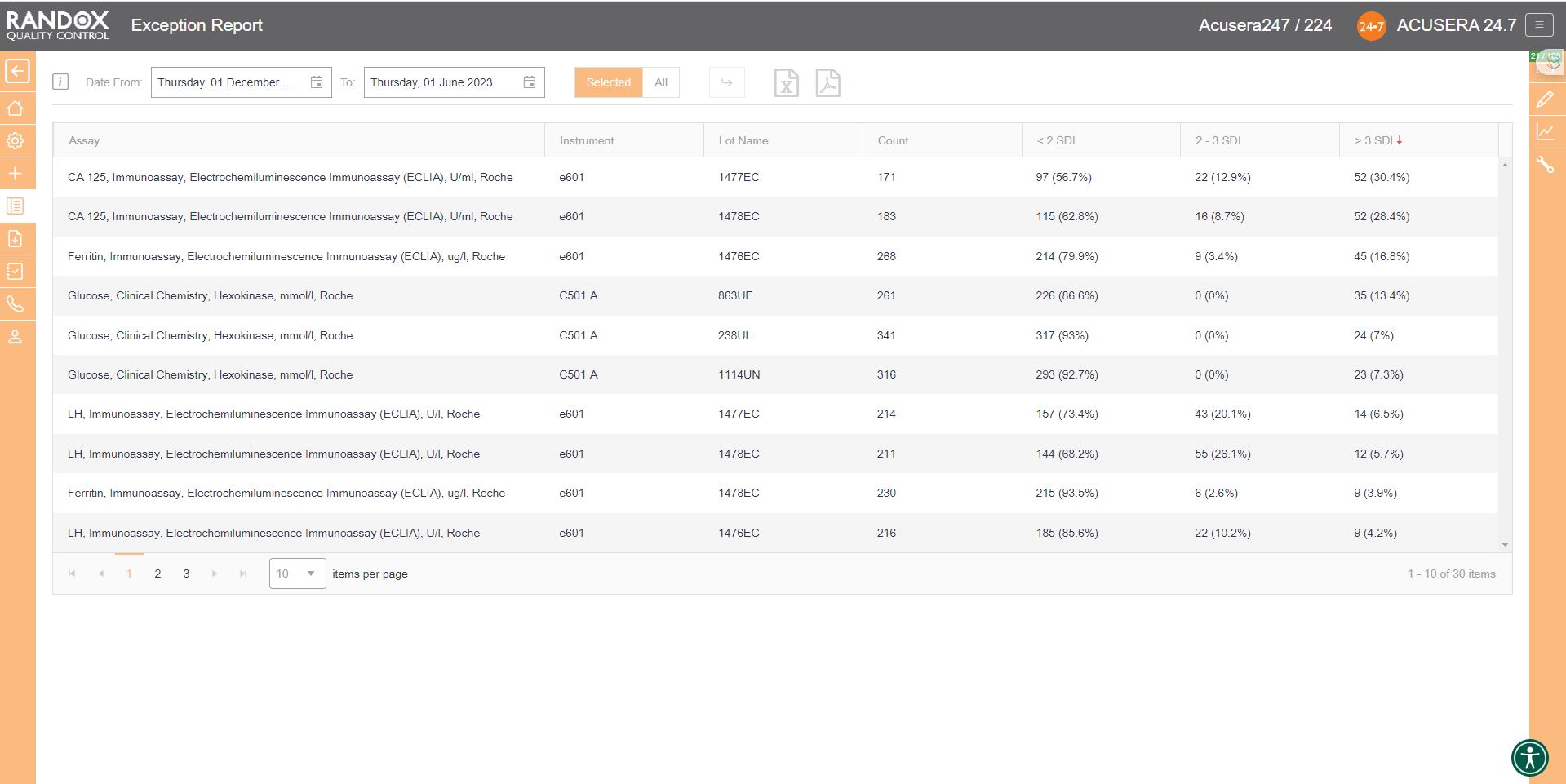
Filtering by ‘<2SDI’, it will display the same data with your best-performing assays at the top.
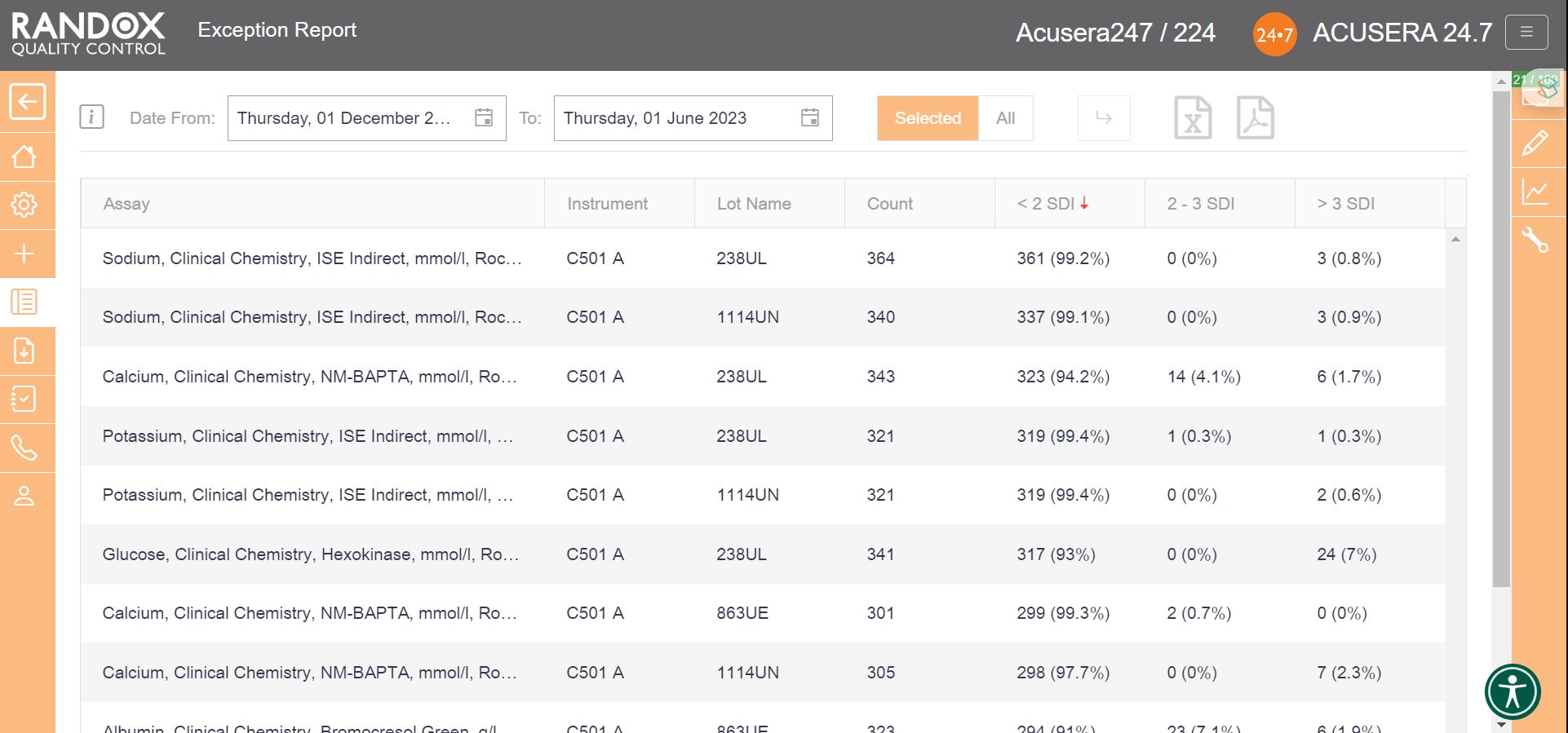
With this information, you can determine in which of your assay’s failures most often occur and encourage staff to look a little more closely at why failures arise and identify changes to improve and minimise errors.
Peer Group Statistics Reports
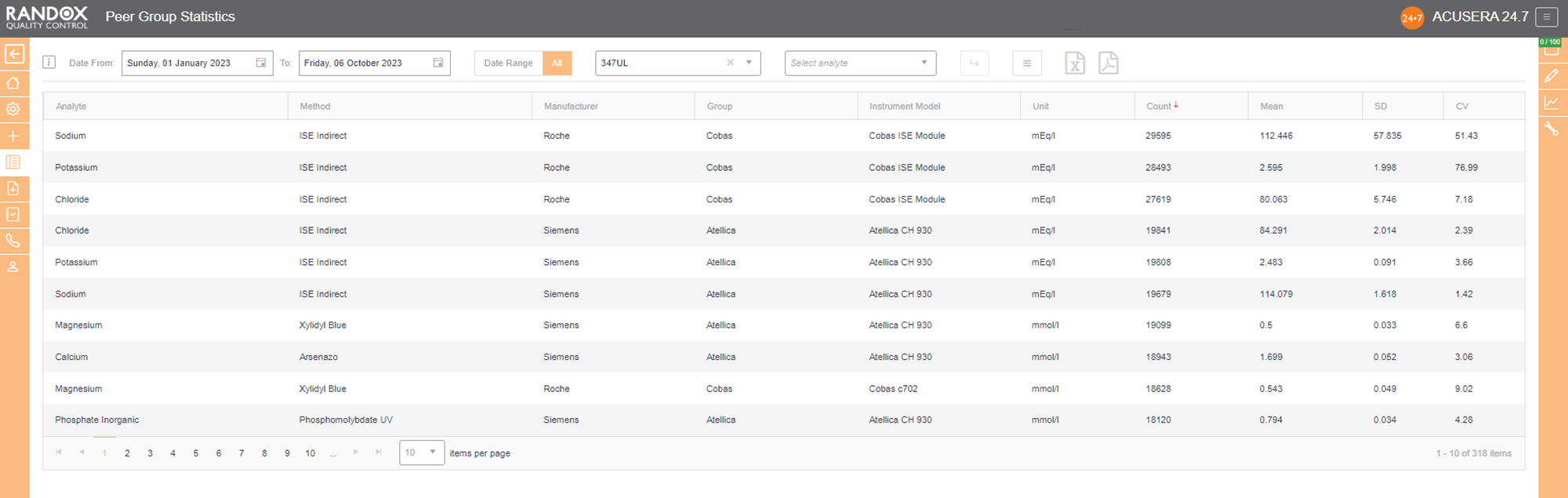
Now that you have figured out the performance of your assays, you’ll want to see how you compare with others running the same tests. Our Peer Group Statistics Report is your new best friend.
Updated live and in real-time, with no submission deadlines, you can compare your statistics to those of your peer group, determined by analyte, method, instrument manufacturer and model.
Simply select the IQC lot you wish to analyse and Acusera 24.7 will generate the data for you, displaying the count of QC data, mean, SD and CV, giving you comprehensive insight into your performance vs your peers.
You can customise this report even further. If you select an analyte, we’ll show you the data for that analyte alone. If not, we’ll show you the information for all analytes related to that lot. The same goes for specifying a date range – if you choose a range, we’ll show you the data inside that range alone. If not, we’ll show you all the data for your chosen lot.
By clicking on the headers, you can filter the data – 1 click will display the data in ascending order, 2 will show you a list in descending order and 3 clicks will reset the table.
When these reports are combined with the other impressive features of Acusera 24.7, like our fully customisable charts or advanced statistical analysis, this software can help streamline your IQC procedure and data review process.
When the accreditors come knocking, others will be scuffling around trying to gather multitudes of reports and files, but you will be sitting with a smile on your face and your feet up, because you’ve got Acusera 24.7.
With full onboarding assistance and technical support that’s top-of-the-class, you’ll always have someone to help you get to the bottom of any problems that you face.
If you haven’t already booked a demo, get in touch with us today and let us show you how much time we can save you with this innovative and intuitive software. Alternatively, take a look at our Resource Hub for some material on Acusera 24.7 or Acusera IQC.
To streamline your QC Data analysis, get in touch with us at marketing@randox.com.
Acute Kidney Injury and Antimicrobial Stewardship
An estimated 1 in 5 hospital admissions in the UK is associated with acute kidney injury1, providing a clear illustration of the need for novel, rapid detection methods. Our latest whitepaper looks at this common condition and the links between Acute Kidney Injury and Antimicrobial Stewardship. For more details on the things discussed in this article, you can download the full whitepaper below.
Acute Kidney Injury
Acute Kidney Injury is defined as a sudden loss of kidney function. This causes a disruption in the kidneys’ ability to filter waste out of your blood resulting in an accumulation of waste products as well as other imbalances.
The loss of kidney function is the result of a sudden reduction in glomerular filtration rate (GFR), the process through which waste is extracted from the blood and is often reversible2.
Aetiology of Acute Kidney Injury
The differential pressure existing between the glomerulus and Bowman’s is the driving mechanism for glomerular filtration2. This pressure contrast is influenced by the combined resistances of the afferent (leading to the glomerulus) and efferent (leading away from the glomerulus) vascular pathways in the kidney. Under normal kidney function, these resistances are in equilibrium, facilitating the proper functioning of the GFR. For example, an increase in efferent resistance restricts the blood flow out of the kidney, elevating pressure inside the kidney and reducing GFR, and vice versa2. However, in AKI, the decline in renal blood flow and GFR has a pathological origin. The pathophysiology of AKI can be classified as prerenal, intrinsic renal, or postrenal.
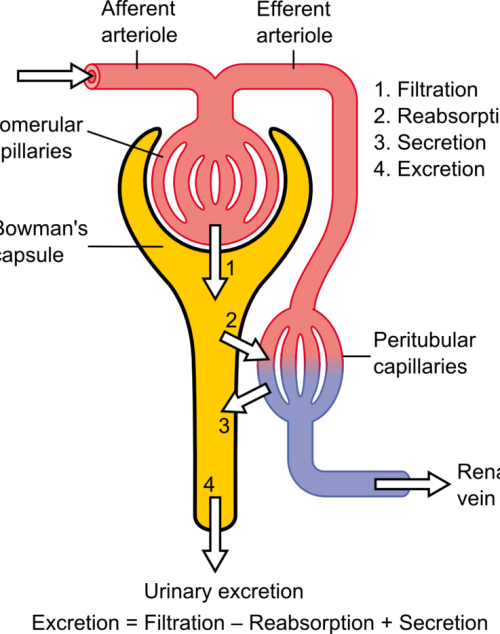
Pre-renal Acute Kidney Injury
Pre-renal AKI is caused by reduced afferent blood flow or, in other words, increased afferent resistance. While tubular and glomerular functions generally remain intact, pre-renal AKI may be caused by systemic hypoperfusion (decreased blood flow) or selective hypoperfusion to the kidney, caused by renal artery stenosis or aortic dissection3.
Intrinsic Renal Acute Kidney Injury
Renal AKI describes the conditions which affect the glomerulus or tubule, for example, acute tubular necrosis and acute interstitial nephritis. This collection of conditions is associated with vasoconstrictor expression in renal afferent pathways2.
Post-renal Acute Kidney Injury
Post-renal AKI usually results from an obstruction in the filtration system. Causes of obstruction include kidney stones, tumours, or blood clots, commonly in the bladder outlet. Obstruction affecting one side might not invariably lead to acute kidney injury, especially when the impediment develops slowly, such as with tumour growth. This is because the unaffected kidney might be able to adjust and make up for the compromised functionality3
Symptoms & Treatment
AKI often manifests with decreased urine output as its primary symptom. However, additional symptoms, when present, can encompass fatigue, nausea, vomiting, or confusion4. To achieve an accurate diagnosis, a comprehensive review of the patient’s medical history and a physical examination are essential to identify the underlying cause of the condition.
The treatment and management of AKI are contingent upon the root cause of the condition. In milder cases, measures are implemented to maintain appropriate levels of fluid, electrolytes, and blood pressure. Nutritional support may also be administered when necessary. In the most severe instances of AKI, dialysis may be warranted to compensate for the diminished kidney function5.
Creatinine serves as a valuable diagnostic tool for evaluating renal conditions, including kidney health, GFR, and muscular dystrophy. However, abnormal serum creatinine (SCr) levels only become evident when a significant portion of the renal mass is compromised. The kidneys possess an impressive capacity to adapt to reduced function, which means that a considerable loss of function or GFR is necessary to influence SCr levels. This poses a challenge when it comes to early detection of AKI6.
Novel biomarkers, KIM-1, NGAL, Clusterin, and Cystatin C, are associated with AKI2 and can be analysed through molecular testing. These new methods can provide a fast and accurate assessment of an individual’s kidney health, at a much earlier stage than SCr quantification2.
Antimicrobial Stewardship
Antimicrobial Stewardship (AMS) programs are specifically crafted to enhance the efficiency of antimicrobial utilization, curtail the emergence of Antimicrobiasl Resistance (AMR), and enhance patient outcomes7. These programs encompass a variety of approaches, such as educational initiatives, training, the establishment of guidelines and protocols, ongoing monitoring and feedback regarding antimicrobial usage, and the management of antimicrobial formularies. Through the promotion of prudent antibiotic utilization, AMS programs contribute to the safeguarding of the efficacy of currently available antimicrobial agents and the deceleration of AMR development7.
Antibiotics and Acute Kidney Injury
Various antibiotics are associated with the progression of AKI due to their nephrotoxicity which can cause severe damage to the kidneys. These antibiotics include polymyxins, aminoglycosides and the commonly used, vancomycin8.

Randox Renal Injury Detection
Using the patented Biochip Technology, the Randox Acute Kidney Injury (AKI) array, available on the Evidence Investigator, simultaneously tests for four novel biomarkers (KIM-1, NGAL, Clusterin, Cystatin C) delivering an early diagnosis and monitoring of treatment efficacy. Multiplex testing better captures reduced renal function, as each biomarker reflects different mechanisms that result in similar injury outputs, allowing for a more accurate picture of the underlying cause of AKI. Along with being able to identify AKI at a much earlier stage, this array provides an accurate and sensitive solution for the diagnosis and monitoring of AKI.
If you’d like some more information on the Randox Acute Kidney Injury Array or would like to add this technology to your laboratory, take a look at our website at https://www.randox.com/acute-kidney-injury/ or get in touch today at marketing@randox.com.

References
- NICE. How common is it? Acute Kidney Injury . Published July 2023. Accessed October 2, 2023. https://cks.nice.org.uk/topics/acute-kidney-injury/background-information/prevalence/
- Adiyanti SS, Loho T. Acute Kidney Injury (AKI) Biomarker.; 2012.
- Manzoor H, Bhatt H. Prerenal Kidney Failure.; 2023.
- NHS. Acute Kidney Injury. NHS. Published 2023. Accessed July 31, 2023. https://www.nhs.uk/conditions/acute-kidney-injury/
- Goyal A, Daneshpajouhnejad P, Hashmi M, Bashir K. Acute Kidney Injury . In: StatPearls [Internet]. StatPearls Publishing ; 2023.
- Rule AD, Lieske JC. The estimated glomerular filtration rate as a test for chronic kidney disease: Problems and solutions. Cleve Clin J Med. 2011;78(3):186-188. doi:10.3949/ccjm.78a.11004
- Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990-1001. doi:10.1016/S1473-3099(17)30325-0
- Clifford KM, Selby AR, Reveles KR, et al. The Risk and Clinical Implications of Antibiotic-Associated Acute Kidney Injury: A Review of the Clinical Data for Agents with Signals from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) Database. Antibiotics. 2022;11(10):1367. doi:10.3390/antibiotics11101367
National Cholesterol Month
National Cholesterol Month
Foe or Friend? Your Health is your Wealth.

It’s National Cholesterol Month, time to raise awareness on the dangers high cholesterol can have on your body and the strain it can have on your precious heart, the centre piece and vital organ that keeps you alive and pumps blood to every corner of your body.
Cardiovascular disease is the leading killer, claiming 17.9 million lives globally each year. 80% of these are due to strokes and heart attacks. Its not too late to get checked out and to alter your lifestyle habits.
Randox Laboratories provides a comprehensive range of reagents for Cardiovascular disease with sdLDL providing detailed testing of cholesterol levels and overall Cardiovascular Health, see more in the blog below.
Cholesterol can be a friend, but it can also be a foe. Maybe you associate bad cholesterol with the result of being overweight, this is not always the case, without a balanced diet, essential exercise, you are at risk of being in the cholesterol danger zone.
Small dense Low-Density Lipoprotein (sdLDL) carries cholesterol to and from cells in the body, it is one of two proteins. However, it is more atherogenic than LDL cholesterol meaning it has a higher tendency to leave fatty deposits in the blood and has a greater ability to block arteries.
Low-density lipoprotein (LDL) is key for progression and development of cardiovascular disease and plague build up (atherosclerosis). LDL has a couple of subclasses sdLDL being one, making it a more reliable marker for the discovery and testing of cardiovascular issues.

sdLDL has a significantly greater atherogenic potential than LDL sub-group. This makes the portion of sdLDL a better marker to predict cardiovascular disease and issues than LDL. It provides a greater understanding of lipoprotein risk within patients; it is more comprehensive in detecting cardiovascular risk in comparison to the original LDL-C test. It is a valuable screening tool in allowing to detect for diseases and abnormalities in the cardiovascular system. As sdLDL-C is particularly atherogenic, a person with elevated sdLDL-C levels has a 3-fold increased risk of myocardial infarction.
Randox provides the “only direct automated sdLDL-C kit on the market, The Randox sdLDL-C ‘Ex-Seiken’ test is a direct method for the quantitative determination of sdLDL-C using automated chemistry analysers, capable of accommodating two-reagent assays. The assay consists of two steps and is based on the use of well-characterised surfactants and enzymes that selectively react with certain groups of lipoproteins.”
Benefits include,
- Direct, automated test for convenience and efficiency
- Rapid analysis results can be produced in as little as ten minutes, facilitating faster patient diagnosis and treatment plan implementation.
- Good correlation to the gold standard ultracentrifugation method
- Liquid ready-to-use reagents for convenience and ease of use
- Applications available detailing instrument specific settings for a wide range of analysers
- Clearance method sdLDL-C controls and calibrator available.
Foe or Friend? Your Health is your Wealth.
References
Fernandez-Alvarez R, Gonzalez-Rodriguez AP, Gonzalez E, Rubio-Castro A, Dominguez-Iglesias F, et al. Serum Ferritin as Prognostic Marker in Classical Hodgkin Lymphoma Treated With ABVD-based Therapy. Leukemia & Lymphoma . 2015;56(11):3096-3102.
Randox (2023) Ferritin: Reagents, Randox Laboratories. Available at: https://www.randox.com/ferritin/ (Accessed: 20 September 2023).
Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Medicine . 2013;11(185).
Sharma J, Sharma R. A prognostic marker in patients with sepsis in pediatric age group: A prospective cohort study. International Journal of Medical and Health Research . 2018;4(3):86-89.
The Royal College of Pathologists. Guidance on the Use and Interpretation of Clinical Biochemistry Tests in Patients with COVID-19 Infection.; 2020. Accessed September 18, 2023. https://www.rcpath.org/uploads/assets/3f1048e5-22ea-4bda-953af20671771524/G217-RCPath-guidance-on-use-and-interpretation-of-clinical-biochemistry-tests-in-patients-with-COVID-19-infection.pdf
World Health Organisation. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations.; 2020. Accessed September 18, 2023. https://www.who.int/publications/i/item/9789240000124



