What is Measurement of Uncertainty?
What is Measurement of Uncertainty?
Measurement Uncertainty (MU) relates to the margin of doubt that exists for the result of any measurement, as well as how significant the doubt is. For example, a piece of string may measure 20 cm plus or minus 1 cm, at the 95% confidence level. As a result, this could be written: 20 cm ±1 cm, with a confidence of 95%. Therefore, we are 95% sure that the piece of string is between 19 cm and 21 cm long.
Standards such as ISO 15189 require that the laboratory must determine uncertainty for each test. However, they have not specified how this should be done.
How do we calculate Measurement Uncertainty using QC data?
Employing your QC data to calculate uncertainty makes several assumptions; your test system is under control, the patient samples are treated in the same manner as your controls and gross outliers have been removed. If you choose to use your QC data to calculate this you should ensure that you use a commutable control with a matrix similar to that of a patient sample, with analytes present at clinically relevant levels
To calculate MU, labs must look at the intra-assay precision and inter-assay precision of their test.
Intra-assay precision: Sometimes known as ‘within run’ precision, is where 20 or more replicates of the same sample are run at the same time, under the same conditions (calculated from a single experiment). Intra-assay precision helps to assess systematic uncertainties
Inter-assay precision: Sometimes known as ‘between run’ precision, is where 20 or more replicates are run at different times – e.g. 1 replicate every day for 20 days (can be calculated from routine IQC data). Inter-assay precision can help identify random uncertainties within the test system.
*The Australian Association of Clinical Biochemists (AACB) recommends that at least 6 months’ worth of QC data are used when calculating the inter-assay precision1.
Once the data is collected, you must calculate the standard error of the mean (SEM) of the intra-assay precision (A) and the SD of the inter-assay precision (B) in order to measure the uncertainty (u). Once A and B have been calculated, they need to be squared, added together and the square root of the sum found:
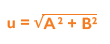
As uncertainty is calculated as SD and 1SD is equal to 68% confidence on a standard Gaussian curve, we can conclude that if we multiply using a coverage factor of 2, we can attain 2SD confidence of 95%. This is known as the Expanded Uncertainty (U):
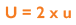
What is the Advantage of Measurement Uncertainty for a lab?
Labs need to carry out MU as it is a requirement of ISO 15189. It states: “The laboratory shall determine measurement uncertainty for each measurement procedure, in the examination phases used to report measured quantity values on patients’ samples. The laboratory shall define the performance requirements for the measurement uncertainty of each measurement procedure and regularly review estimates of measurement uncertainty”.
MU also helps determine whether the difference between two results is negligible due to uncertainty or significant due to a genuine change in condition of the patient; giving labs a greater confidence in reported results.
How can Randox help?
Our new Acusera 24.7 Live Online software provides automatic calculation of MU, saving valuable time and helping labs meet ISO 15189 requirements with ease.
Contact marketing@randox.com to find out how your lab can benefit from Acusera 24.7 Live Online
Take steps to prevent incorrect patient results by making one simple change
According to the NHS Litigation Authority; in 2015 within the UK alone, £193,680,744.30 was spent on ‘wrong diagnosis’ or ‘failed/delayed diagnosis’ causing huge financial strain and impact on labs.
With approximately 75% of clinical decisions and diagnosis based on laboratory test results. The only way to guarantee a high degree of accuracy is to implement a good Quality Control plan. The importance of this is recognised globally, several bodies exist internationally including ISO (International organisation for standardisation) who have developed a set of guidelines and quality systems to ensure the reliability of laboratory test results.
So what can you do to improve accuracy and reliability?
Choose a third party QC
ISO 151589:2012 Section 5.6.2.2 states that “the use of third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer”.
First Party Controls are those manufactured by the instrument/reagent manufacturer. These controls are optimised specifically for use with the manufacturers test system and therefore will mask a multitude of weaknesses. First Party Controls tend to result in perceived accuracy and a biased assessment of performance.
Third Party Controls on the other hand are designed to be completely independent and are not optimised for use with a specific test or system. Leading manufacturers of third party controls will assign target values based on data collected from thousands of independent laboratories, ensuring the availability of statistically robust multi-method, multi-analyser data. Therefore laboratories using Third Party Controls can be assured of unbiased error detection across multiple platforms.
Randox Acusera is a world leading manufacturer of true third party controls providing a cost effective, high quality solution for any laboratory-regardless of size or budget.
Look out for QC samples with clinically relevant concentrations
ISO 15189:2012 states that ‘The laboratory should choose concentrations of control materials wherever possible, especially at or near clinical decision values, which ensure the validity of decisions made’.
It is important to assess the full clinical range of an assay i.e. the range between the lowest and highest results which can be reliably reported. In order to make sure a laboratory instrument is performing accurately across the full clinical range and in particular at the medical decision level, QC materials that cover low, normal and elevated concentrations should be used.
Due to the superior manufacturing process used by Randox, QC target values consistently cover the MDL of tests. By ensuring the controls in use cover clinical decision levels laboratories can be confident of the reliability and accuracy of the patient results they release.
Opt for a commutable control material
A good QC material has many essential properties but above all, controls must perform consistently and reflect the performance of patient samples – if a control meets these requirements then we can say it is commutable. Having a commutable control would aid in the prevention of incorrect patient results because they replicate the performance of a patient sample and react to the test system in a similar manner. Use of a commutable control will also reduce costly shifts in QC target values when reagent batch is changed.
At Randox we take quality seriously, that’s why all QC products are manufactured to the highest possible standard, delivering controls of unrivalled quality. Designed to be commutable, the Acusera range will ensure accurate and reliable instrument performance while simultaneously helping laboratories to meet ISO 15189:2012 requirements. A good QC process will include the use of Third Party Controls, Clinically Relevant Concentrations and controls which can be described as commutable. By employing Quality Control’s that encompass these traits, a laboratory professional can be certain that they have taken the necessary steps to decrease incorrect results and therefore potential misdiagnosis.
Aliquoting for longer QC stability
Al-i-quot: An amount that is an exact divisor of the whole quantity of a substance (Collins Dictionary of Medicine, R. Young, 2005).
Why aliquot QC material?
Aliquoting QC material can extend the open vial stability of a lyophilised control, according to manufacturer recommendations. By splitting your QC material into a number of tubes and freezing these you can extend the working stability of the control, ultimately reducing wastage and the amount of money spent on unnecessary additional controls.
Example
A laboratory purchases a lyophilised QC with a volume of 3ml once reconstituted the control is stable for 7 days at 2-8oC. However, the laboratory only uses 1ml of this control per week, meaning that 2ml could potentially be wasted. The manufacturer states that the control can be frozen after reconstitution, extending the working stability from 7 days at 2-8oC to 30 days at -20 oC to -80oC. The following outlines the process for aliquoting reconstituted material and extending the control’s working stability.
Aliquoting reconstituted material
- Reconstitute the QC material according to the manufacturer’s instructions.
- Using a micropipette aliquot the required volume (generally a minimum of 0.5ml should be used) of reconstituted material into a tube.
- Repeat step 2 until all the reconstituted material has been aliquoted.
- Label each tube with the date the material was reconstituted to avoid the use of expired material.
- Store each aliquot at -20oC in a frost free freezer. Be sure to check the kit insert for frozen stability claims.
- Remove and thaw each aliquot as and when required making sure to use all material within the frozen stability period.
- Once thawed do not refreeze, dispose of any leftover QC material.
Conclusion
Aliquoting reconstituted material is an ideal way of extending the control’s open vial stability. This will ensure that your laboratory minimises the amount of QC material wasted and saves money by eliminating the need to purchase additional controls. Please note that not all lyophilised controls can be frozen like this. To ensure the controls you are selecting are suitable for aliquoting check the product’s kit insert or contact your supplier.
What can Randox Quality Control offer?
We have a number of lyophilised controls which can be prepared and stored in this way across our extensive product portfolio. To find out more visit www.randoxqc.com or contact us via acusera@randox.com to arrange a visit from one of our QC Consultants.
The Benefits of Peer Group Data to your Troubleshooting Process
Drive for more accurate results in your laboratory
We’ve all been there, you’re in the middle of a run of patient tests when you are alerted to an out of control event, such as your analyser is reporting QC results 25% low to target. What do you do? In reality, we all know that the problem is unlikely to correct itself, especially if it’s a calibration or analyser issue. Human error is a potential factor, however all possible causes must be eliminated to proceed with patient testing.
What’s the solution?
ISO 15189:2012 recommends that a laboratory should “have a procedure to prevent patient results in the event of a quality control failure”. Implementing an interlaboratory data management program which features peer group reporting can help you meet this requirement and monitor the results you are producing. Such programs can help detect errors in the analytical phase of patient testing, through the automatic application of pre-programmed QC rules, thus alerting staff to failed results.
Why must Peer Groups be a feature?
A peer group is defined as a “Community in which most or all members have roughly the same characteristics…” (Businessdictionary.com, accessed 2017). In this instance the characteristics could refer to the; instrument, test method or QC material in use. As such peer group programmes could help you detect errors in your laboratory by comparing your results to those who are employing a similar method, instrument and QC to what you are using, i.e. comparing apples for apples. Therefore it is essential that the peer group data you require is available in real-time, to ensure you are accessing the most up-to-date data when reviewing your patient test results.
Scenario
Take the example from the introduction. You’re in the middle of a run of patient tests when you are alerted to an out of control event, such as your analyser is reporting QC results 25% low to target. As part of your troubleshooting procedures, you are able to compare your results to the results of your peer group and note that this is an isolated incident. Consequently, you have eliminated a widespread problem with the QC, reagent or calibrator and narrowed down the root cause to one of the components in your test system. Thus saving you time in the troubleshooting process.
Benefits of Peer Group Comparison
There are a number of benefits to employing peer group comparison in your laboratory. Peer group data comparisons facilitate faster troubleshooting, helping you identify whether the problem you are seeing is unique to your laboratory, or if other laboratories are reporting the same issue. If other laboratories are reporting the same issue it is possible to conclude that there is a widespread problem with either the QC, reagent or calibrator. On the other hand, if it is not occurring within your peer group you will have to investigate further, reviewing your QC processes. As a result, you could resolve issues much quicker by eliminating either a supplier or laboratory issue. Furthermore, you can also eliminate the need for unnecessary repeat tests or instrument maintenance, saving both valuable time and money.
Other characteristics you should look out for
Whilst peer group comparison is a useful feature there are a number of other features you should consider when selecting the right interlaboratory data management program for you. These include;
- Automatic calculation of Measurement Uncertainty, Total Error and Sigma Metrics
- Multiple laboratory management on a single platform
- Accessing data anytime, anywhere via PC, laptop or tablet via a web-based platform
- All data charts you may require to assess whether any bias or imprecision issues are present
- Ability to combine data for multiple QC lots, analytes and instruments on a single Levey-Jennings or Histogram chart
- Automated data import via a direct connection to your LIMS
What can Randox offer?
At Randox we are passionate about quality control and believe in producing high-quality material that can streamline procedures for laboratories of all sizes and budgets through our Randox Quality Control brand. Acusera 24.7 Live Online is just one aspect of our extensive laboratory portfolio that has been designed to help you produce results you can trust. With Acusera 24.7 Live Online you can drive for more accurate results by monitoring and interpreting QC data online, anytime, anywhere. With access to an impressive range of features, including the automatic calculation of Measurement Uncertainty, Total Error and Sigma Metrics, Acusera 24.7 will ensure analytical quality.
The RX series – Precision. Reliability. Accuracy

Precision
The RX series analysers ensure that the on-board testing process is precise through many stages and therefore lead to accurate results in the laboratory. Our automated analysers go through an extensive washing system for cuvettes and includes acid, alkali and pure water wash steps. Liquid level sensor, crash, bubble and clot detection ensure that after the washing process is complete, whatever residue remaining has been completely removed from the cuvettes so that they are ready to be used again.
Reliability
Randox understand the demands of the clinical chemistry laboratory and recognise the importance of maintaining a consistent workflow of high quality results. We pride ourselves in excellence of service. Through our global network, our team of trained engineers provide local service and support. There are 3 levels of service maintenance provisions to ensure a package to suit all laboratories; bronze, silver and gold packages.
Randox also offers technical support 24 hours a day, 7 days a week via a free electronic service called Powerline; this service provides customers with instant access to instructions for use (IFU), and instrument specific application (ISA) documents for a comprehensive list of instruments. Each RX series analyser requires minimal maintenance, as little as 5 minutes daily, and between 1 and 2 yearly preventative maintenance checks depending on the workflow of the analyser.
Accuracy
70% of medical decisions are based on laboratory results, therefore it is imperative that accurate results are produced in the laboratory. These test results lead to key decisions for the treatment of patients, therefore it is essential for an accurate diagnosis of all patient samples. A misdiagnosis can be life threatening, or lead to incorrect treatment plans delaying the recovery of the patient, which in turn can lead to high compensation costs for Hospitals and Laboratories for the affected patients.
The RX series analysers ensure accurate test results with a stringent validation process of all Randox Reagents. The QC functionality provides automatic flagging of inaccurate results and testing errors therefore helping laboratories identify and resolve issues quickly, minimising the possibility of misdiagnosis.
Precision, reliability and accuracy are hugely important within a laboratory. The RX series analysers offer these in abundance. Randox can provide consolidation of testing with validated reagents and cost effective Quality Controls, ensuring that your laboratories testing results are precise, reliable and accurate.
If you are interested in finding out more and would like to speak with a Randox representative, please contact us by emailing therxseries@randox.com
Acusera 24•7 Live Online – Speeding up the Review of QC Data
Reviewing QC data can be an extremely time consuming and costly process. With manual statistical calculation laboratories risk missing or ignoring significant trends in QC data which could potentially put patients at risk. So how does a laboratory combat this? Simple; participate in an interlaboratory data management program that provides a quick, effective, accurate and detailed analysis of QC results. The answer to this program is Acusera 24•7 Live Online.
Acusera 24•7 Live Online
With the launch of Acusera 24•7 Live Online version 2.0, QC data review is now faster and simpler than ever before. Our program aims to save the laboratory precious time and money by instantly flagging any QC failures, ultimately ensuring accurate test system performance.
Designed to complement and be used primarily with our Acusera range of true third party controls, Acusera 24•7 Live Online has two primary functions; 1) management and interpretation of IQC data and 2) rapid and effective troubleshooting of QC failures via access to instantly updated worldwide peer group statistics.
These two functions have one common goal – being an effective tool for evaluating laboratory performance. With the launch of version 2.0 the software boasts even more functionality than before, ensuring any laboratory employing Randox Quality Control coupled with Acusera 24•7 Live Online will see benefits from the get-go.
Why should you use Acusera 24•7 Live Online?
Using Acusera 24•7 to help speed up the review process in your laboratory can reap dividends. The program has been designed for this specific reason and the features are geared towards helping the laboratory review, interpret, and analyse QC data quickly, effectively and accurately. One such example of this is the unique dashboard function which instantly flags any alerted or rejected results from the past 7 days, significantly reducing the time spent analysing reports and charts whilst simultaneously allowing any corrective action to be taken immediately with minimum disruption to the lab’s output.
Previously, peer group statistics would have been updated every 24 hours with Acusera 24•7 Live Online version 1.6, however, with the new release, peer data is about to get a unique upgrade. Gone are the days when you will have to wait 24 hours to get updated stats – Acusera 24•7 Live Online now has the ability to generate peer data live in real-time, thereby enhancing the laboratory’s troubleshooting capabilities and allowing labs to compare their data with others around the globe. What’s more there is no deadline for submission of results meaning labs can get a true reflection of performance at any time. Ultimately, laboratories will be able to easily identify if an issue is unique to them or a widespread issue amongst their peers. Such information will allow them establish a root cause quicker and spend less time troubleshooting.
The capacity to generate interactive charts and comprehensive reports automatically is a feature included in Acusera 24•7 that will aid quick review of QC data. Reports can be generated for a user-defined date range and provide a wealth of information. Reports include statistical analysis, statistical metrics, measurement uncertainty, exception and audit trail reports. Reports coupled with Levey-Jennings, Histogram and Performance Summary charts enable rapid and stress-free performance monitoring. The ability to add multiple instruments, QC lots and analytes to a single chart allows for comparative performance assessment and immediate identification of any trends.
We must not forget that Acusera 24•7 Live Online has already had a modernisation in the past few months. In November 2016 we announced the automatic calculation of Measurement of Uncertainty, Total Error and Sigma Metrics. These new features are also included in the version 2.0 launch of our Live Online program.
Our software is highly flexible with custom configurations of performance limits, multi-rules and target values designed to meet and exceed every laboratory’s needs.
With the ability to identify trends, system errors, minimise false rejections and bridge the gap between IQC and EQA, there really is no reason to look elsewhere for your analytical performance of QC.
For more information on Acusera 24•7 Live Online or our Acusera third party controls, click here.
Celebrate Christmas with Randox Quality Control
T’was the week before Christmas and all through the lab not a thing could be heard not even a sound. The analyser lay silent asleep in the corner, the lab staff at home dreaming of a few days’ rest, only a few more days to go before the big day!
The big man in red, what will he bring those who already have everything? Peace, happiness and health for their loved ones throughout the festive break, that would be the wish for everyone to make. And what better way to ensure they stay healthy, well it all begins in the laboratory…
An important consideration to remember when choosing your lab Quality Control (QC) is that approximately 70% of clinical decisions are based on laboratory test results. It is therefore essential that the results gained from laboratory testing are accurate and reliable in order to provide the appropriate treatment and avoid or prevent potential misdiagnosis.
Patient results are of the utmost importance for a laboratory and therefore running the best Quality Control material should be at the top of their agenda. QC material should have a number of features that allow a lab to judge the overall quality of their output. These features include the controls ability to be commutable (which means how well it reacts as a replicate of a patient sample), is it a true third party control that has been manufactured to provide an independent and unbiased assessment of performance, does your control come with clinically relevant levels and does it have a long shelf life as well as a good open vial or reconstituted stability? These are the questions lab staff will be asking themselves when deciding on what QC is the right QC.
So stay off Santa’s naughty list by providing accurate and reliable patient test results, do this by employing Randox QC in your laboratory. Our controls have been designed to deliver significant cost savings without sacrificing on quality. With consolidated controls (combining up to 100 analytes in a single vial) your lab can reduce QC costs and preparation time, the inclusion of analytes present at clinical decision levels will eradicate the need for additional controls and because of our long shelf life (2 years for liquid controls, 4 years for lyophilised) and excellent stability claims your laboratory can be sure that expensive lot changes will be a thing of the past! Our controls can be described as true third party and this, combined with the commutable nature of the controls, leads to us being able to claim that we have the best Quality Control material around.
So this Christmas when deciding what QC to choose – make sure you look no further than Randox Quality Control. Our QC family is known as Acusera and our product offering includes QC and calibrator material, Interlaboratory Data Management Program (Acusera 24.7), the world’s largest international EQA/PT scheme better known as RIQAS and the newest addition to the family, Linearity or Calibration Verification material.
We have packages for every lab regardless of size and budget and we guarantee you will become ho-ho-hooked on Randox QC.
Wishing you all season’s greetings and a prosperous New Year from everyone at Randox QC.

QC Shelf Life – Why is it Important?
An important consideration when choosing your Quality Control material that is often overlooked is the shelf life of the control. With every new lot of control extensive validation studies must be performed. Regulatory bodies such as CLIA require new lot numbers to be evaluated before routine use in the laboratory. For example, CLIA has instructed that any new control lot to be run alongside patient samples will need to be verified alongside the old lot of control. The process is designed to give laboratory professionals confidence in the new material and ensure it is fit for purpose before implementing it in the lab.
As part of the validation process laboratories are required to assay both the old and new lots side by side. The current lot is then used to help verify if the new lot will be acceptable to run within the lab. Such validation studies can be very costly for a lab as well as being extremely time consuming – with some studies taking up to a month to complete! By choosing a control with a longer shelf life laboratories can aim to use the same lot of control for a longer time period. Ultimately this means fewer lot changes and minimal inconvenience for the lab. With a shelf life of 2 years for liquid controls and up to 4 years for lyophilised, coupled with unrivalled stability claims, employing Randox Quality Control in your laboratory will ensure that expensive lot changes will be a thing of the past. Our comprehensive control offering is guaranteed to increase efficiency and reduce costs in any laboratory without compromising on quality.
Contact us today to find out more information on our Acusera range of Quality Controls.
ISO 22870- Are you meeting expectations?
Quality control has recently become crucial in the Point-of-Care (POC) field due to the introduction of ISO 22870 regulations and increased focus in patient safety. Quality control is critical in reducing turnaround time and saving money.
There is now an international standard specifically for POC testing, ISO 22870. This standard is intended to be used in conjunction with the standard for medical laboratories, ISO 15189. This means that aspects relating to Point-of-Care such as training, competence and documentation should be carefully planned, implemented and governed by a quality management system and there is a requirement for QC and EQA to be performed, where available.
POCT is typically carried out by non-laboratory staff, therefore when selecting the appropriate IQC material for POCT there are a number of key characteristics you must consider;
- Format of the material – QC material employed should be liquid stable, requiring no preparation, reducing the likelihood of human error and increasing convenience.
- Value assignment – all values must be accurately assigned. Look out for suppliers who use a large number of independent labs to determine the target value.
- Third party controls – manufactured independently from any specific instrument or method third party controls are designed to deliver unbiased performance assessment.
- Storage – for convenience controls should be liquid stable, as these can be easily stored in a fridge at 2oC – 8oC and won’t need to be shipped on dry ice.
- Stability – a control with a good open vial stability will mean that it can be used for longer with less waste produced, meaning it is more convenient for the medical professional to use.
- Transportation– the liquid stable controls can be conveniently stored at 2oC – 8oC reducing the need to ship on dry ice
- Minimal training– easy to use with little training required, therefore suitable for use by non-laboratory personnel
In addition to IQC, External Quality Assessment (EQA) must also be employed to ensure a comprehensive review of test system performance. It is best to select a programme that offers frequent reporting with a large database of users. This will enable rapid error identification and ultimately accurate and reliable patient testing.
Our Acusera liquid ready-to-use controls include:
- Blood Gas Control– A liquid stable control provided in easy to open ampoules for added convenience and ease-of-use. Assayed, method specific target values are provided for the most common blood gas instruments.
- Liquid Cardiac Control– This is a highly convenient liquid stable cardiac control offering excellent consistency. Assayed, instrument specific target values are provided for 8 cardiac markers, enabling flexibility and consolidation.
- Liquid Urinalysis Control– Liquid control that is compatible for use with both manual and automated methods of dipstick analysis. Available in convenient 12ml vials or 25ml dropper bottles with assayed ranges provided for 13 parameters covering the chemical examination of urine specimens.
- Liquid HbA1c Control– This is another highly convenient liquid ready-to-use control. With an open vial stability of 30 days, keeping waste and costs to a minimum.
Complementary EQA programmes are also available to meet the needs of ISO 22870.
IQCP: Where Are We Now?
What is IQCP?
IQCP stands for Individualized Quality Control Plan, and it is an all-inclusive approach to creating a customized quality control plan for a laboratory.
IQCP focuses on assuring quality in the lab using more in-depth means than simply carrying out a certain number of QC tests at a specific frequency. Many different aspects of laboratory operations will be evaluated, such as the test system, reagents, environment, testing personnel etc.
Where are we now?
As of January 2016, many labs in and outside the USA have implemented their IQCP’s, but what impact has this had on day-to-day operations?
In order to gauge the overall effectiveness and user-experience of implementing IQCP, Westgard QC1 conducted a survey for all IQCP participants both in the USA and globally.
Opinions were mixed regarding the effectiveness of IQCP:
Positive Opinions of IQCP
- Some users found that IQCP decreased the number of QC materials required
- There is a greater emphasis on the pre and post-analytic phases of testing, thus improving process error identification
- Over half of global survey participants revealed that their IQCP identified unacceptable risk(s) in their test system, thereby creating a more robust process
- Of the labs whose IQCP’s were inspected in the USA, 96.3% were deemed adequate by the relevant regulatory bodyies
- Identification of errors can lead to additional personnel training, thereby increasing the knowledge and expertise of laboratory staff
Negative Opinions of IQCP
- Due to the length of time taken to create a single IQCP, coupled with the additional expense, several survey participants found that the benefits of IQCP did not justify using so many resources in its implementation
- Many labs raised concerns regarding the availability of guidance in developing an IQCP. Participants complained that useful guidelines were not provided quickly enough, and labs had to rush their IQCP implementation.
- Several survey participants felt as though there was widespread confusion over IQCP. Participants highlighted that the volume of questions from laboratory professionals proves that IQCP was not introduced by regulatory bodies in an organized or effective manner
- Some labs surveyed voiced the opinion that IQCP evaluation needs to be more standardized, and that inspections can either be too lenient or too stringent.
ISO 15189:2012 requirements
As with any new system, feedback is important for further refinement. IQCP appears to be a step in the right direction for the advancement of laboratory QC. According to Westgard’s survey1, only around 30% of US respondents were satisfied, showing that labs still feel improvements need to be made. Inspectoral standardization, or more concise, straightforward guidelines on IQCP implementation could be potential improvements for regulatory bodies to consider.
We would love to know your thoughts on the subject. Send us an email at acusera@randox.com.
References:
-
Westgard QC. (2016).2016 IQCP Users Survey. Available: https://www.westgard.com/iqcp-user-survey-comments.htm. Last accessed 25-Oct-16.








