Norbrook partners with Randox on staff screening programme for COVID-19
Norbrook partners with Randox on staff screening programme for COVID-19
Pharmaceuticals company Norbrook Laboratories has revealed that it will be the first company in Northern Ireland to install an innovative COVID-19 testing platform for on-site staff screening.
The Newry-based firm, which specialises in the development of pharmaceuticals for the veterinary industry, will begin testing for its workforce in the coming weeks, to provide reassurance and peace of mind for colleagues and for their families, without impacting on the public health need.
The COVID-19 screening programme at Norbrook is being facilitated by diagnostic technology from global diagnostics company Randox, one of the partners within the national COVID-19 testing programme.
David Ferguson, Managing Director at Randox Food Diagnostics explained;
“Collectively we are all working towards a timely return to a more normal society, which will see companies reopening and people returning to work.
“To facilitate this recovery of the economy, without compromising the health of workers or of the wider general public, workplaces have a responsibility to provide a safe working environment.
“We know that the health of Norbrook’s staff is their priority and as such it is great to see them taking a proactive approach to testing.”
The COVID-19 screening programme at Norbrook is the first in Northern Ireland being facilitated by an innovative testing platform capable of processing results in 2hours 30 mins.
The technology, named the Vivalytic, can screen for SARS-CoV-2, the virus that causes COVID-19, as well as a range of other viral and bacterial infections including Influenza A and B, Pneumonia and other coronaviruses.
David added;
“Randox is fully committed to supporting the national effort to fight COVID-19 by testing at scale, and as we continue to work alongside the government and ramp up our testing capabilities, we welcome the fact that other organisations are also adopting our innovative COVID-19 testing technologies to address their own particular testing needs.
“The Vivalytic, which provides high quality molecular testing for COVID-19, on-site and without the need for laboratory experience, is a unique space-saving, hygienic solution for personnel COVID-19 testing in any setting and will help a wide variety of industries get back to business by ensuring the highest level of safety.”
Denise Collins, Norbrook Human Resources Director, concluded;
“We’re proud to be working with a company like Randox which has such vast experience in the diagnostics industry and was subsequently able to respond so quickly to the COVID-19 pandemic.
“That this world-leading technology is available from a Northern Ireland-headquartered company presents a unique opportunity for workplaces here to quickly and easily implement a staff screening programme, and at the same time demonstrate a high standard of duty of care that should be shown by employers to their employees.”
For further information about the Vivalytic please email marketing@randox.com
Want to know more about Randox?
Contact us or visit our homepage to view more.
Our Randox Products and Services
REAGENTS
RX SERIES
ACUSERA
BIOCHIP
Whole pathogen molecular controls for SARS-CoV-2 (COVID-19) receive CE marking
25 May 2020
Whole pathogen molecular controls for SARS-CoV-2 (COVID-19) receive CE marking
CE marking has been granted to whole pathogen quality controls for SARS-CoV-2 (COVID-19), from global diagnostics company Randox Laboratories.
The molecular controls are currently being used alongside the firm’s pioneering COVID-19 tests, performed on its proprietary Biochip, as part of a nationwide UK testing programme.
Randox is using these third-party quality controls, which include a Positive Control, Negative Control, Analytical Q Panel and Molecular Q Panel, to ensure accurate coronavirus test and system performance, and ultimately, guarantee laboratory quality assurance.
Lynsey Adams, Randox Quality Control Manager, explained;
“Accurate and reliable laboratory testing is an essential aspect of COVID-19 disease management and outbreak control.
“Designed specifically for laboratories currently managing COVID-19 testing, Randox SARS-CoV-2 controls, which have been CE marked, help ensure the accuracy and reliability of molecular assays used in the testing of suspected coronavirus samples, and importantly, give clinicians absolute confidence in any COVID-19 diagnoses they may make.”
Available under the brand name Qnostics, the new SARS-CoV-2 controls are manufactured using whole pathogens. They therefore mimic the patient sample, providing the best sample matrix for laboratories.
Lynsey continued;
“The Randox Qnostics SARS-CoV-2 controls are clinically relevant for full-process validation. Liquid frozen for user convenience and ease of use, the development of the controls using whole pathogen material ensures clinical relevance from extraction to amplification and detection.”
The new coronavirus controls from Randox, which contain the entire SARS-CoV-2 genome, including the conserved regions recommended by CDC and WHO, are compatible for use with both commercial and in-house testing methods, and are quantified by digital PCR to ensure batch to batch reproducibility.
Lynsey added;
“We are proud to be able to support the high demand for coronavirus testing by delivering a full COVID-19 testing package – not only our Coronavirus Biochips but now also the corresponding molecular control material that will ensure the delivery of accurate, and reliable results.
“The World Health Organization (WHO) has been very clear about the importance of testing in the global efforts to contain and delay COVID-19 and we are pleased that our new CE-marked controls comply with guidelines not only from the WHO but also from the Centres for Disease Control and Prevention.”
Randox Qnostics SARS-CoV-2 Controls are heat-inactivated to be non-infectious and are manufactured to ISO 13485 standards.
Key Features and Benefits of Randox SARS-CoV-2 Qnostics Controls:
- Whole pathogen – the controls contain the entire SARS-CoV-2 genome meaning they are compatible with the majority of commercial and in-house assays, and target the CDC and WHO consensus sequences
- Monitor the entire testing process – whole pathogen controls are the ideal clinically relevant material for full-process validation, from extraction to amplification and detection, to ensure ultimate quality assurance in laboratories.
- Non-infectious – the controls are heat-inactivated and gamma-irradiation enabling safe handling of material.
- Highly characterised – the controls are quantified by digital PCR to ensure batch to batch reproducibility.
- Clinically relevant – performance data is available to support the clinical relevance and the compatibility with molecular assays currently in use in clinical laboratories.
- High Quality – the controls are manufactured under ISO 13485 guidelines and are therefore suitable for a broad range of usage.
- Liquid for Ease-of-Use – conveniently supplied in a liquid frozen format meaning there is no additional preparation or handling required.
- Complete QC package – Analytical and Molecular Q Panels available for new assay validation as well as routine performance monitoring.
For more information visit www.randox.com/coronavirus-randox
QNOSTICS
QCMD
CORONAVIRUS
Randox calls upon NI engineers to build COVID-19 testing platforms
RANDOX CALLS UPON NI ENGINEERS TO BUILD COVID-19 TESTING PLATFORMS
Randox Laboratories has today announced it is recruiting 160 mechanical, electrical and manufacturing engineers to enhance its capacity for COVID-19 testing.
The engineers, who are due to begin work at the Randox Science Park from the middle of May, will be involved in the fast-tracked development of specialist molecular analysers used to detect the presence of COVID-19.
These testing platforms will be used to further enhance the government’s national testing scheme for key workers, as well as to facilitate testing more broadly across the general populace.
Dr Peter FitzGerald, Managing Director of Randox Laboratories, commented;
“It is Randox’s priority to ensure that we support the UK’s effort to fight COVID-19, by testing at scale. We know that this is the most effective way to both save lives and promote a timely return to a more normal society.
“We continue to ramp up our COVID-19 testing capabilities – not only by increasing our production of testing kits, but also by accelerating the build schedule for the testing analysers on which the tests are performed.
“We are aiming, by working at maximum efficiency in a greatly accelerated time frame of 6-7 weeks, to manufacture 200 of our most state-of-the-art testing platforms, which will greatly enhance our testing capacity at Randox.”
Randox has, in recent weeks, established a number of new teams responsible for the development, manufacturing and distribution of COVID-19 testing kits for the national testing programme.
The company is also interested in speaking to anyone with experience in mechanical, electrical or manufacturing engineering. Invest Northern Ireland is helping by contacting companies which may be able to temporarily release resources to help meet this urgent staffing requirement.
Dr FitzGerald added;
“To meet the unprecedented demand for COVID-19 testing, we have been redeploying our own personnel to various COVID-19 critical departments, but are now also recruiting for a number of specialist disciplines.
“Everyone at Randox has a vital role to play in the practical application of COVID-19 testing, and for engineers in particular, this is a unique opportunity to make a positive impact in the fight against COVID-19, by directly contributing in no small part to the national testing programme.”
Anyone interested in the Randox COVID-19 Engineering Drive should apply at careers.randox.com
Want to know more?
Contact us or visit our Randox Careers
Find out more about Randox Careers
Careers News
Vacancies
Our People
Careers Website
Health Secretary Matt Hancock publicly thanks Randox for ‘stepping up to the mark’
Health Secretary Matt Hancock publicly thanks Randox for ‘stepping up to the mark’
On 23 April 2020, UK Health Secretary Matt Hancock personally thanked staff at Randox for the work we have done and continue to do, during the daily coronavirus briefing from Downing Street.
He said;
“I want to take this moment to applaud the private companies who have been involved…Boots, Amazon, Thermo Fisher, Randox, Rosch, Oxford Nanopore, GSK and Astrazeneca. They have really stepped up to the mark and I am grateful for each and every one.”
It is Randox’s priority to ensure that we contribute to the global effort to fight COVID-19, by testing at scale. We are therefore absolutely committed to the government testing scheme, which is focusing on key workers and the maintenance of critical national infrastructure – including that of the NHS.
In order to meet the requirements of this scheme, we have undertaken considerable reorganisation within the company by redeploying personnel – often to newly formed departments and newly formed teams. Everyone at Randox has a critical role to play in the practical application of COVID-19 testing and we appreciate all the hard work, patience, and flexibility of our staff.
Our work has greatly expanded the capacity for testing in the UK and we will continue to ramp up our capabilities, to both save lives and promote a timely return to a more normal society.
What we are doing is making a real and positive difference.
For further information please email randoxpr@randox.com
To watch the full version of the Daily Coronavirus Briefing from 23rd April, please click here.
Want to know more about Randox?
Contact us or visit our homepage to view more.
Our Randox Products and Services
REAGENTS
RX SERIES
ACUSERA
BIOCHIP
Randox unveils whole pathogen molecular controls for SARS-CoV-2 (COVID-19)
16 April 2020
Randox unveils whole pathogen molecular controls for SARS-CoV-2 (COVID-19)
Whole pathogen quality controls to support accurate coronavirus testing have been developed by scientists in the UK.
The molecular controls, available from global diagnostics company Randox Laboratories, are currently being used as part of a nationwide testing programme for frontline NHS workers.
Alongside its pioneering COVID-19 tests, performed on its proprietary Biochip, Randox is using these third-party quality controls to ensure accurate coronavirus test and system performance, and ultimately, guarantee laboratory quality assurance.
Lynsey Adams, Randox Quality Control Manager, explained;
“Accurate and reliable laboratory testing is an essential aspect of COVID-19 disease management and outbreak control.
“Designed specifically for laboratories currently managing COVID-19 testing, Randox SARS-CoV-2 controls help ensure the accuracy and reliability of molecular assays used in the testing of suspected coronavirus samples, and importantly, give clinicians absolute confidence in any COVID-19 diagnoses they may make.”
Available under the brand name Qnostics, the new SARS-CoV-2 controls are manufactured using whole pathogens. They therefore mimic the patient sample, providing the best sample matrix for laboratories.
Lynsey continued;
“The Randox Qnostics SARS-CoV-2 controls are clinically relevant for full-process validation. Liquid frozen for user convenience and ease of use, the development of the controls using whole pathogen material ensures clinical relevance from extraction to amplification and detection.”
The new coronavirus controls from Randox, which contain the entire SARS-CoV-2 genome, including the conserved regions recommended by CDC and WHO, are compatible for use with both commercial and in-house testing methods, and are quantified by digital PCR to ensure batch to batch reproducibility.
Lynsey added;
“We are proud to be able to support the high demand for coronavirus testing by delivering a full COVID-19 testing package – not only our COVID-19 tests on the Randox Biochip, but now also the corresponding molecular control material that will ensure the delivery of accurate, and reliable results.
“The World Health Organization (WHO) has been very clear about the importance of testing in the global efforts to contain and delay COVID-19 and we are pleased that our new controls comply with guidelines not only from the WHO but also from the Centres for Disease Control and Prevention.”
Randox Qnostics SARS-CoV-2 Controls are heat-inactivated to be non-infectious and are manufactured to ISO 13485 standards.
Key Features and Benefits of Randox SARS-CoV-2 Qnostics Controls:
- Whole pathogen controls – the controls contain the entire SARS-CoV-2 genome meaning they are compatible with the majority of commercial and in-house assays, and target the CDC and WHO consensus sequences
- Monitor the entire testing process – whole pathogen controls are the ideal clinically relevant material for full-process validation, from extraction to amplification and detection, to ensure ultimate quality assurance in laboratories.
- Non-infectious – the controls are heat-inactivated and gamma-irradiation enabling safe handling of material.
- Highly characterised – the controls are quantified by digital PCR to ensure batch to batch reproducibility.
- Clinically relevant – performance data is available to support the clinical relevance and the compatibility with molecular assays currently in use in clinical laboratories.
- High Quality – the controls are manufactured under ISO 13485 guidelines and are therefore suitable for a broad range of usage.
- Liquid for Ease-of-Use – the controls are conveniently supplied in a liquid frozen format meaning there is no additional preparation or handling required.
- Negative controls available – delivering a complete testing package.
For more information visit www.randox.com/coronavirus-randox
QNOSTICS
QCMD
CORONAVIRUS
Official statement from Randox on Covid-19 testing
03 April 2020
OFFICIAL STATEMENT FROM RANDOX
On the emergence of the Covid-19 threat Randox utilised their years of regulated diagnostic experience to quickly develop and robustly validate a test for the Covid-19 virus. Randox had significant confidence in this test and presented them to Public Health England (PHE) for evaluation. PHE were managing this validation process for each of England, Wales, Scotland and Northern Ireland.
The UK Government showed significant confidence in Randox’s capability and conducted full engagement and planning subject to PHE acceptance of the Randox test. When PHE acceptance was granted, the Randox part in the national plan had been prepared and was quickly initiated.
We understand that the national plan for the testing of key workers is exactly that, a national plan, inclusive of Northern Ireland. Initially relatively small numbers of tests were sent to the areas of significant national threat in London.
The planning for the national distribution of test kits is being managed by the various relevant statutory agencies however Randox has made the case that tests should be made available locally. Following that engagement, tests have now been made directly available within Northern Ireland and Randox will continue to support Northern Ireland within the UK national plan.
Randox have acted with speed and initiative throughout this crisis and will continue to ramp up our testing capability to support the national testing plan for key workers in Northern Ireland.
Every part of Randox’s business is focused on doing everything we can to support the Covid-19 testing programme, to both save lives and ensure the speediest possible return to a more normal society.
For more information please contact the Randox PR team by emailing randoxpr@randox.com
QNOSTICS
QCMD
BIOSCIENCES
Randox joins government drive on COVID-19 tests for frontline NHS staff
27 March 2020
Randox Laboratories is partnering with the government to develop a new coronavirus testing programme in which NHS staff will be first in line.
The new service, which will be free, will help to end the uncertainty of whether NHS staff need to stay at home. Those who test negative for coronavirus will be able to return to work – enhancing the capacity of the NHS and social care to treat patients and care for those in community settings, with plans for a full roll-out for health, social care and other frontline workers.
Randox are providing high volume home sample collection kits and laboratory testing.
Randox CEO Dr Peter FitzGerald, said:
“We have an excellent Covid-19 assay and we are committed to supporting the government and this important initiative to test key workers and support vital public services.
“Randox Laboratories is now operational for COVID-19 testing and will be ramping up extensively in the coming weeks. This will provide extensive employment opportunities for laboratory and support staff.
“Our first tests have gone to the London Ambulance Service and we look forward to playing our role in ensuring that this critical capability continues to operate.”
To offer its coronavirus testing capabilities to the new programme, Randox’s COVID-19 test recently underwent evaluation from, and has been accepted by, Public Health England.
In its evaluation of the Randox COVID-19 test, Public Health England’s report noted that the assay correctly identified all positive and negative samples without exception.
As a result the government are proceeding to utilise the Randox COVID-19 test in the new testing programme for NHS staff, with an exceptionally high degree of confidence.
Two simultaneous tests are carried out on the proprietary Randox Biochip, in line with guidelines from the Centres for Disease Control and Prevention, and the World Health Organisation.
Health Secretary Matt Hancock said:
“We want to save lives, protect the most vulnerable, and relieve pressure on our NHS.
“Healthcare staff are key in our fight against the virus and I want to ensure that any frontline NHS or care worker who has symptoms of coronavirus or who has a family member with symptoms can be tested quickly and reliably.
“I pay tribute to the generosity and public spirit of Britain’s universities, research institutes and companies who have lent us their equipment without hesitation.”
Dr Jenny Harries, Deputy Chief Medical Officer, said:
“Laboratory-based testing on this scale is a little like building the medical equivalent of a car factory. We are assembling many different parts, some of them quite specialised and hard to find, then getting them to work accurately together in a highly co-ordinated process. There are bound to be teething problems, so we cannot switch on hundreds of thousands of lab tests overnight. But we hope that soon these hub laboratories will be operating round the clock, allowing us to significantly scale up our testing.”
For more information or to arrange interviews, please contact the Randox PR team by emailing randoxpr@randox.com
QNOSTICS
QCMD
BIOSCIENCES
Point of care coronavirus test from Randox and Bosch will launch in April 2020
26 March 2020
Vivalytic Viral Respiratory Tract Infection Array Detecting SARS-CoV-2 (COVID-19)
A game-changing point of care coronavirus test from global diagnostics company Randox Laboratories and leading technology manufacturer Bosch Healthcare Solutions will launch in April 2020.
The Vivalytic Viral Respiratory Tract Infection (VRI) Array can identify SARS-CoV-2 (COVID-19) and differentiate it from nine other respiratory infections with similar symptoms, including influenza and all known coronaviruses.
This provides a more comprehensive respiratory screening which enables precise and informed treatment decisions to be made.
Dr. Heather McMillan, Molecular R&D Manager at Randox Biosciences, commented;
“This array focuses not only on the identification of the novel coronavirus strain that causes COVID-19, but also nine other respiratory infection targets simultaneously, including Influenza A and B, Sarbecovirus and MERS. The aim is to diagnose patients fast and accurately from a single genetic sample, maximising containment of the virus whilst minimising the spread. With the development of this array onto a fully automated platform, patients can be diagnosed rapidly at point of care locations globally such as pharmacies and doctor surgeries.”
The Viral Respiratory Tract Infection Array is one of the world’s first multiplex molecular diagnostic tests meeting the COVID-19 testing recommendations of the World Health Organisation (WHO) and the Centres for Disease Control (CDC).
The target genes for COVID-19 being used on the VRI array represent conserved regions of the genome which have been chosen for their high sensitivity and specificity.
Marc Meier, Managing Director of Bosch Healthcare Solutions, a subsidiary of Bosch Group, said:
“We are enthusiastic about partnering with Randox to offer their assay technology on the Vivalytic platform. The core competencies of Bosch in automation, miniaturization, sensor technology and connectivity are complemented by Randox’s expertise in developing excellent biocontent for a wide range of assays and commercializing innovative diagnostic solutions.”
The new VRI test will be conducted on Vivalytic a point of care platform brought to the market by Randox Laboratories and Bosch. The Vivalytic system is a fully-automated, cartridge-based platform capable of both Hi-Plex and Lo-Plex testing. The cartridges are fully-sealed which minimises the risk of contamination, require room temperature storage (space-saving), contain all the reagents on-board the cartridge and utilises end point PCR. The test only requires a single nasal swab from the patient and an easy four step process to be carried out by the user to run the patient sample.
Key Benefits of Vivalytic | Point of Care Platform
- Fully-automated
- User friendly
- No laboratory training required
- 4 step work flow
- Minimal contamination risk – fully contained system
- No peripherals required – hygienic
- Touchscreen
- Connects to LIMS (Laboratory Information Management System)
- Sample Type: Nasopharyngeal Swab
- Sample Volume: 300µl clinical sample
For more information or to arrange interviews, please contact info@randoxbiosciences.com
RESEARCH
BIOPHARMA
CLINICAL LAB
BIOREAGENTS
Drug Use in Australia: A Randox Toxicology Report
19 March 2020
Drug Use in Australia
Drug Use in Australia: A Randox Toxicology Report
Approximately 43% of all Australians aged 14 years and over have admitted to using an illicit drug on at least one occasion throughout their life.
Additionally, nearly 16% of Australians have confessed to using an illicit drug at least once in the last year.
Cannabis is the most frequent drug used among the Australian people with an estimate of 35% lifetime use. The closest to this is MDMA which has an estimate of around 11% lifetime use.
A reason towards the high cannabis use may be due to the Australian Capital Territory legalizing the recreational possession and cultivation of marijuana in September 2019. This now allows any residents of the capital territory over the age of 18 to possess up to 50 grams of dried marijuana and grow two plants per person or four per household at a time. The supply of the drug to other people however still remains an illegal act.
It is estimated that approximately 60 per cent of those who use illicit drugs are also daily smokers and/or consume alcohol at a level that is considered to be of risk to their everyday health. Another noticeable finding among drug users in Australia is that the majority of methamphetamine, cocaine or ecstasy users are also users of other illicit drugs.
Using our revolutionary Biochip Array Technology, the Evidence MultiSTAT is a fully automated benchtop analyser that enables onsite, simultaneous testing for up to 21 classical, synthetic or prescription drugs from a single sample in under 20 minutes.
To find out more about our Biochip Array Technology and our Evidence Series range of analysers, visit www.randoxtoxicology.com or email info@randoxtoxicology.com
Randox Toxicology Products and Services
BIOCHIP
EVIDENCE
ELISA
MATRICES
Want to know more about Randox?
Contact us or visit our homepage to view more.
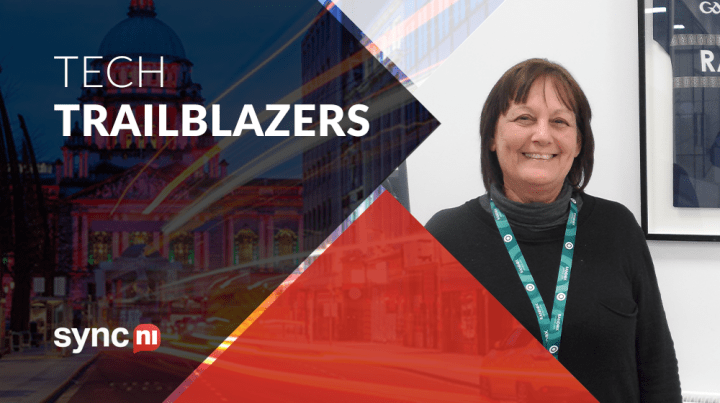
We Are Randox | Tech Trailblazer Margaret Le Roux
Tech Trailblazers: Margaret Le Roux
Sync NI, in celebration of Women in Tech, recently spoke with our IT Operations Team Leader Margaret.
Read on as Margaret share’s her typical day, the favourite thing about her job, and how her specialist role at Randox combines a degree in Biomedical Science with a passion for software development.
Name: Margaret Le Roux
Role: IT Operations Team Leader, Randox Laboratories
Biography:
I graduated from the University of Rhodesia (now known as Zimbabwe), as a Biomedical Scientist in 1980. My career in South Africa was then predominantly in the clinical laboratory medicine field, and I qualified as a Technical Assessor for clinical laboratories through the South African National Accreditation System. I moved to Belfast in 2014 and started work with Randox.
What does your typical day look like?
I work in an IT Operations role bridging our science and quality control software, which assesses the accuracy and reliability of blood tests, and the machines they are run on, in the likes of hospitals, laboratories, and veterinary clinics. On a typical day, I deal with customer queries about this software, troubleshoot the issues, and drive new developments to improve our systems and applications. I also spend time training Randox staff, mentoring some of my junior colleagues, and speaking at Biomedical Science Conferences to educate others in the industry about the importance of quality control software.
What are you currently working on?
I’m currently working on the specification for our external quality assessment software, which involves a mock blood sample being run in a laboratory’s analyser, and the result being sent back to Randox so that we can independently check that it is performing correctly.
I have also been working on the software project that saw Randox win ‘Project Team of the Year’ at the 2019 Belfast Telegraph IT Awards. This cloud-based quality control technology was specifically designed for ‘Point of Care’ machines which provide finger prick blood tests for conditions like heart disease and diabetes, in pharmacies, GP surgeries and A&E departments.
Did you always want to work in the tech industry?
I have been very lucky that my specialist role at Randox combines my degree in Biomedical Science, and my experience in QC, with my passion for software development. In South Africa, I worked as a Quality Officer for a large private laboratory, with 3 main laboratories and over 100 peripheral sites across 10 African countries. The management of quality control data was a huge job and as such I became interested in a software program that could assist the lab with this task.
What inspired you to join Randox in particular?
Whilst working in South Africa, I was one of Randox’s customers, and made extensive use of their quality control products. Randox has always had a very good reputation in South Africa so when I moved to Belfast it was a natural choice for me.
What’s your favourite part about your work?
It’s a great feeling when we introduce a new release to our software and you know that the customers are going to benefit from it.
What would you say to other people considering a job in the tech industry?
A job in the tech industry is simultaneously exciting and challenging, as each day brings something new. You will continually be making improvements and striving to make something better, which is a good work ethic. It’s really satisfying when you are part of a team which develops a software program that is so well accepted in the market and useful to the customer.
How do you see this technology impacting on our lives?
The technology industry is so fluid and moving at such a fast pace, and there are developments across all industries which are making our lives easier. At Randox in particular, our software is helping a range of healthcare professionals – whether laboratory technicians, clinicians or veterinarians – to achieve our shared goal of saving and improving the lives of patients. It’s rewarding to know we are making a difference.
Who inspired you to work in this field?
In the field of Quality Control, I was inspired by Dr Pandelani Rambau, a Clinical Pathologist from Johannesburg. In IT, it was a colleague Sean Dicks who showed me that there is always a way to get a program to do what you need it to do.
What do you consider to be the most important tech innovation or development in recent years?
The development of communication devices has been incredibly important. They open up the whole world to us and we can access things, both socially and for education, that previously were only available to a few. They have brought so much information to our fingertips.
What tech gadget could you not live without?
I couldn’t live without my phone, because it is so much more than just a phone. It holds all the important things that make up my life, like messages, memories, and my calendar.
To find out more about Randox IT and the vacancies we have in the team, please email recruitment@randox.com
For more We Are Randox stories about our amazing colleagues, make sure to follow us on Facebook, Instagram and Twitter and follow the hashtag #WeAreRandox.
Want to know more?
Contact us or visit our Randox Careers






