The Randox Grand National Trophy (2024) is revealed.
The Randox Grand National Trophy (2024) is revealed.
Friday, 29th March
The Randox Grand National Trophy is one of the most iconic and prestigious sporting trophies in the world of horseracing.
From the start of the Randox sponsorship in 2017, Randox CEO Dr. Peter FitzGerald proposed a unique trophy would be designed and produced each year – the winning owner receiving a full-sized trophy, with the winning trainer, jockey and groom all receiving a miniature version.
Each trophy is uniquely Randox and has a story to tell. The design on the trophy has evolved over the years. Innovation is a characteristic that Randox takes great pride in. Randox is dedicated to improving health using innovative diagnostic technologies. This year’s design was the innovative work of talented De Montfort University placement student, Ritik Tailor.
On his design, Ritik says, “I am very fortunate to be given the opportunity to design the Randox Grand National Trophy. Being entrusted with such an important design at the start of my placement is quite rewarding. With the support of the team, I was able to create a trophy that feels like a major accomplishment for me as an intern. It’s also an incredible opportunity as the trophy is being presented to the winning jockey at the Randox Grand National. It is a great feeling that something I have created will be woven into the history of such a prestigious sporting event.”
This year’s trophy has two key elements -the design showcases the elegance of a horse mid-jump with nods to Randox’s expertise in biochemistry. A double helix, representing DNA, rises from the base of the trophy and is partially encased by two silver rods that act as its handles.
The horse, rising from a circular blood drop – is one of the most iconic symbols of the event, was something Ritik considered an essential component to incorporate into the design. “I wanted to capture the racehorse in a way that would make it stand out as the focal point of the trophy.” adds Ritik, “After brainstorming a couple of sketched, I decided on a dynamic and lifelike horse sculpture.”
But the life-like horse sculpture is not the only element that makes the trophy unique.
The Randox iconic logo is incorporated into the base in a subtle, yet impactful way – perfectly complimenting the horse. To tie everything together the DNA Helix design is another key feature.
“I wanted to incorporate Randox’s expertise in the field of biochemistry and molecular diagnostics into the design. This intricate design is etched into the metal base of the horse, symbolizing Randox’s icon.”
Graphic Design Team Lead, Elizabeth Heaney adds, “Ritik has truly made his mark within our design team during his placement. His trophy design being chosen over others from multiple designers is a testament to his creativity and talent. We couldn’t be prouder of this years Randox Grand National trophy – and I hope Ritik feels the same sense of accomplishment for his involvement in its creation.
“It’s amazing to think that Ritik accomplished this feat within just four weeks of joining Randox, demonstrating his quick adaption to the company and his ability to create a design that embodies Randox’s essence flawlessly!”
The Randox Grand National trophies over the years have been brought to life by silversmith, Cara Murphy who adds, “This is the fifth year I have had the honour of making the Randox Grand National trophy. It is always exciting to see the different designs and how they each celebrate the event.”
Overall, the Randox Grand National Trophy is beautiful and intricate work of art that embodies the company’s commitment to innovation, accuracy, and precision in the field of diagnostic testing and opportunity.


RIQAS Performance Assessment – Z Score vs SDI
Z Score vs SDI
You work hard to implement top class quality control in all areas of your laboratory. The success of your labours is reported to you through your External Quality Assessment (EQA) results. It can be frustrating when your report is returned, only for you to find that you’ve been assigned a poor performance score due to other laboratories in your participation group.
At RIQAS, we want your EQA results to reflect your performance, not that of everyone else, to truly illustrate the efficacy of your quality control procedures. This is why, instead of Z scores, we report your performance in terms of a Standard Deviation Index (SDI). However, we know that in some countries, you’re required to report a Z score. Don’t fret. You can still find this result in the .csv file provided with your report.
A Z score is a statistical measurement that describes a value’s relationship to the mean of a group of values. In other words, it’s a value calculated to tell us how many standard deviations (SDs) a result is from the expected mean. Z score is reported in terms of SD’s, therefore a Z score of 0 shows the result is identical to the mean.
While useful in many cases, when used in EQA, a Z score can give a false perception of performance. We want RIQAS participant performance assessment to be based on their individual performance, rather than being impacted by how well, or poorly, the other laboratories in the group performed for a sample.
Z score is calculated using a variable SD. This means that as results are added, the mean and SD can change. For example, if overall performance for a sample improves, the CV associated with the data will decrease, causing an increase in Z score. Let’s take a quick look at how RIQAS performance assessment works, and then we can get into SDI.
RIQAS Performance Assessment.
Our target scoring system has been developed to provide a simple interpretation of your laboratory’s performance. To calculate a target score, your result is calculated as a percentage deviation (V) from the Mean for Comparison. This deviation is then compared to a Target Deviation for Performance Assessment (TDPA) to calculate the Target Score.
The difference between your result and the mean for comparison is expressed as a Target Score (TS) using the following mathematical formulae:

The better your percentage deviation compared to the TDPA, the higher your Target Score will be.

TDPA are set to encourage participants to achieve and maintain acceptable performance. Target Deviations are assigned to be fit-for-purpose and take all possible sources of variation into account, including sample homogeneity and stability as per ISO/IEC17043, ISO13528 and IUPAC.
In general, the TDPA is set so that ~10% laboratories achieve Target Scores less than 50. However, depending on homogeneity and stability, the TDPAs may be adjusted, so that participants’ performance is not adversely affected by sample variability. If your % deviation (V) is equal to the Target Deviation for Performance Assessment (TDPA) then a target score of 50 is achieved.
RIQAS reviews TDPAs annually and the methods used to assign them have been agreed by the RIQAS Advisory Panel.

Standard Deviation Index (SDI)
To provide a more accurate assessment of performance, we use SDI instead of Z score. SDI is a score which compares the participant’s difference from the assigned value (mean for comparison) with an evaluation interval called the Standard Deviation for Performance Assessment (SDPA).
The SDPA calculation involves a series of steps. First, we calculate a CV for Performance assessment (CVPA) as shown below:

As mentioned, the TPDA is normally set so that ~10% of laboratories achieve a TS less than 50. In such cases, the t-value used to convert TDPA to CVPA is ~1.645. However, depending on homogeneity and stability, the TDPA may need be increased, so that participants’ performance is not adversely affected by sample variability. In such cases less than 10% of laboratories will have poor performance, and a larger t-value will be chosen to convert TDPA to CVPA
We then convert CVPA to SDPA:

Using this equation, an initial SDPA is calculated for every mean for comparison (i.e. for all methods, method, and instrument statistics). However, for new parameters or those which have small participation numbers, it’s not always possible to assign a target deviation, TDPA or SDPA. In such cases, the SDPA will be the SD calculated when the mean for comparisons is generated.
According to ISO/IEC17043, when the assigned value is based on consensus (mean for comparison), the uncertainty of the assigned value must be calculated and combined with the SDPA when it is considered to be significant. This forms an adjusted SDPA, which is used to calculate the participant’s performance in terms of SDI.
Using the SDPAadjusted we can calculate SDI using the formula below:

On your RIQAS report, you’ll find the SDI associated with the current sample in the text section of each report page. We also provide your last 20 SDIs, plotted on a Levey-Jennings chart, along with an indication of the mean for comparison for each sample (I = Instrument group, M = Method group, or A = All Methods group). Acceptable performance is an SDI of less than ± 2.

RIQAS EQA
RIQAS is the world’s largest EQA scheme with more than 75,000 laboratory participants spanning over 138 countries. Choosing an EQA provider is no easy task. That’s why we’ve produce a guide to help you find the right one for you. You can download it here.
At RIQAS, we’re always coming up with new ways to make your performance assessment and result interpretation even easier. We’re also proud of our new programmes and pilot schemes. This year, we’re running pilot programmes for Anti-psychotic drugs, Chagas and Blood Typing.
If you’d like to find out more about the range of programmes we provide, visit our website or download our brochure. Alternatively, you can get in touch with us at marketing@randox.com.
Lp(a) Awareness Day 2024
Novel and classical insights into Lp(a) concentration and the effects on various cardiovascular conditions.
Despite advances in understanding and technology, cardiovascular diseases (CVDs) remain a major source of mortality across the world. The World Health Organisation (WHO) estimate that 17.9 million people died due to CVDs in 2019, accounting for around 32% of deaths that year1. First described in 1963, Lipoprotein(a) (Lp(a)) is a macromolecular lipoprotein complex2 which is thought to display proatherogenic, proinflammatory3 and prothrombotic4 potential and is considered an independent causal risk factor for various types of CVD5. These properties provide several mechanisms in which elevated Lp(a) levels may contribute to CVD however the true nature of Lp(a)s relationship to CVD remains largely enigmatic.
Lp(a) concentrations in plasma are principally regulated by variation in LPA gene and levels remain relatively stable throughout one’s lifetime with lifestyle factors having little effect on their concentration6. Due to the highly heritable nature of Lp(a) concentration, those with a family history of Familial Hypercholesterolaemia (FH), elevated LDL-C levels, or Atherosclerotic cardiovascular disease (ASCVD) should be screened, their plasma Lp(a) concentration determined, and their risk of CVD established.
In the last 10 years, there have been many advances in the understanding of this ambiguous lipoprotein which support the causal association with CVD, clarify the established evidence and introduce novel mechanisms of action in relation to Lp(a), shedding light on its obscure pathophysiology. However, there are still diagnostic complications associated with Lp(a) measurement as there is little standardisation in methods of determination5.
Physiology and Genetics
Synthesised mainly in the liver, Lp(a), like LDL, is composed of a lipid centre made of cholesteryl esters and triacylglycerols, surrounded by a shell of phospholipids, free cholesterol, and an apoB-100 molecule. The major difference between other LDL molecules and Lp(a) is the presence of a polymorphic glycoprotein, apo(a), bound to apoB-100 by a single disulphide bond5. It is this apo(a) molecule which contributes to Lp(a)s pathophysiology.
Apo(a) is thought to have evolved from the plasminogen gene (PLG) around 40 million years ago and shares 78-100% sequence homology within the untranslated and coding regions of the fibrinolytic enzyme2. Like plasminogen, apo(a) contains unique domains named kringles5. While plasminogen contains 5 different kringle structures (KI to KV), apo(a) has lost KI to KIII and instead contains several forms of KIV, namely, 1 copy of KIV1 and KIV3-10, 1-40 copies of KIV2, 1 copy of KV and an inactive protein domain at the carboxyl terminus of the molecule7. These hydrophilic subunits are highly polymorphic due to the variation in KIV2 repeats. Individuals may possess two different isoforms of apo(a) one of which will have been passed down from each parent and are expressed codominantly2. These isoforms are dependent on the number of KIV2 repeats they contain2. Isoforms with less KIV2 repeats produce smaller apo(a) isoforms which are found at a higher concentration compared with larger isoforms8 due to the increased rate at which the smaller molecules can be synthesised5. The polymorphisms in KIV2 repeats account for up to 70% of the variation seen in concentration between individuals, with the remainder being attributed to differences in protein folding, transport, and single nucleotide polymorphisms (SNPs)5. SNPs are central in the heterogeneity of apo(a), effecting RNA splicing, nonsense mutations and 5’ untranslated region of the LPA gene resulting in shorter gene translation5,8.

Lp(a) Pathophysiology
Lp(a) is thought to contribute to the risk of CVD through multiple mechanisms. Firstly, Lp(a) molecules display all the same atherosclerotic risk as LDL-C molecules due to their similar fundamental composition, for example, their propensity for oxidisation upon entering the vessel wall, and promotion of atherosclerosis through inflammatory and immunogenic mechanisms 9. However, Lp(a) displays more proatherogenic potential due to the presence of the apo(a) molecule. The structure of apo(a) results in decreased fibrinolysis. Due to its structural similarities, apo(a) competes with plasminogen for binding sites, competitively inhibiting plasminogen, ultimately resulting in reduced fibrinolysis9.
Lp(a) is thought to be a preferential carrier of oxidised phospholipids2 (OxPLs) which covalently bind to apo(a), increase expression of inflammatory proteins, and stimulate the secretion of IL-8 and monocyte chemoattractant protein-1, enhancing its ability to cross the vessel wall9. Some claims require further investigation, however, studies have been carried out which show inhibition of plasminogen activation in the presence of Lp(a)10. It is this indirect mechanism that Lp(a) is thought to conduct its prothrombotic activity8,9.
Clinical Evidence
Many studies have been carried out to determine the association of Lp(a) concentration and CVD risk. Studies such as the Copenhagen General Population Study, the Copenhagen City Heart Study, Dallas Heart Study, and Ischemic Heart Disease Studies provide strong evidence for Lp(a) as a causal risk factor for CVD. Data analysis of the Copenhagen General Population Study reveal that 20% of subjects displayed Lp(a) concentrations of more than 42mg/dl, or around 105nmol/L11, which is considered to result in increased risk of atherosclerotic disease5. It is important to note, there is no accepted conversion factor for converting Lp(a) concentration from mg/dl to nmol/L due to the variability of apo(a) kringles. The unitage will depend on the assay method used5. Another study in a healthcare organisation in Israel showed that Myocardial Infarction (MI) and Coronary Artery Disease was 2.5 times more common in those with high levels of Lp(a) than in the age and sex matched control group3. This study3, along with others5,6,12 describes a linear relationship between Lp(a) concentration and CVD risk, showing at least a 3-fold increase in ASCVD and MI events in adults with Lp(a) concentrations in the top 1% when compared with those in the with concentrations in the bottom 20%3.
The major variation in Lp(a) concentration seen throughout the population, is further evident between ethnicities and sexes. On average, Caucasian subjects display the lowest Lp(a) concentrations, with Black subjects displaying the highest concentrations5. However, the large number of functional variants are consistent across ethnicities suggesting that it is the KIV2 repeats and SNPs that are the major factors contributing to Lp(a) concentration regardless of ethnicity. Lp(a) concentrations are higher in women than men8 with levels increasing post-menopause thought to be caused by a decrease in oestrogen3.
Lp(a) Testing and Screening
The European Atherosclerosis Society (EAS) recommend that all adults are tested at least once in their lifetime to identify individuals who have high levels of Lp(a) and therefore high CVD risk. Screening is also recommended in children who have a family history of Ischaemic stroke, premature ASCVD or high Lp(a) levels in the absence of other identifiable risk factors8. Testing has been associated with reduced mortality rates. This is thought to be because of increased and intensified therapy for those who are identified as high risk due to high plasma Lp(a) concentration6.
There are various assays available for the determination of Lp(a) concentration which vary in accuracy and precision. Many of these assays utilise polyclonal antibodies which recognise different antigenic determinants8. Due to the variability in apo(a) structure and KIV repeats, these assays often overestimate the concentration of large isoforms and underestimate concentration of small isoforms when determining the true Lp(a) levels9. This variation can be partially nullified by using a calibrator series and by selecting a method which is traceable to WHO/IFCC reference material. This allows laboratories to confidently identify individuals considered high risk but may still prove problematic when patients’ results report closer to the assay thresholds.
One study13 compared 5 commercially available Lp(a) assays on an automated clinical chemistry analyser. The assays tested were manufactured by Diazyme, Kamiya, MedTest, Roche, and Randox. The authors show that all the assays tested met the manufacturers claims for sensitivity, linearity, and precision. However, significant bias was observed in 4 out of 5 assays. The only assay which did not display significant bias was the Randox Lp(a) Assay which is traceable to WHO/IFCC reference material. This report highlights the importance of measuring and reporting Lp(a) in molar concentration rather than in mass units to facilitate standardisation and harmonisation in Lp(a) testing13.
Current and Emerging Therapies
Statins are one of the most potent treatments for the primary prevention of ASCVD through the reduction of LDL-C concentration. However, recent studies reveal that statins have no effect on Lp(a) concentration3 and others suggest that statin administration can increase Lp(a) concentration by up to 11%5,9. Nonetheless, EAS do not recommend statin therapy be halted as their strong ameliorative effects on CVD risk are well established and surmount the risk related to increased Lp(a) concentration8.
Niacin (Nicotinic acid) is another established treatment for the reduction of CVD events and act by increasing HDL levels. Niacin can reduce Lp(a) concentration though the reduction of gene expression in a dose-dependent manner5. However, Niacin therapy has not been proven to have beneficial effects on CVD risk8.
A recent metanalysis showed a 26% reduction in serum Lp(a) concentration through treatment with PCSK9 inhibitors. This is thought to be due to a shortage of apoB-100 molecules either because of reduced synthesis or competitive binding with other LDL receptors, resulting in reduced Lp(a) concentration5. Several studies show the efficacy of PCSK9 inhibitors in reducing CVD risk, but this is not yet an approved therapy5,8.
New therapeutic strategies aim to target hepatocytes, the site of apo(a) synthesis, to reduce Lp(a) concentration. Antisense Oligonucleotides (ASOs) inhibit apo(a) mRNA in the nucleus and cytoplasm, ultimately inhibiting Lp(a) secretion5 through the cleavage of the sense strand by ribonuclease H19. While still in clinical trials, ASO therapies show promise in the battle to reduce CVD risk with some studies displaying an overall reduction in Lp(a) concentration of more than 80%9.
Conclusions
There have been major advances in the understanding of Lp(a) pathophysiology in the last 10 years establishing this macromolecular complex as an independent causal risk factor for various forms of CVD, however, more investigation is required to fully understand the mechanisms responsible for this association. With many national healthcare organisations and the EAS recommending universal testing for Lp(a) in adults, more emphasis should be placed on raising awareness of the importance of Lp(a) screening. Finally, more research is needed into therapies which succeed at lowering Lp(a) concentration. While some therapies are in clinical trials, there are currently no approved therapies that achieve this goal.
The Randox Lp(a) assay is calibrated in nmol/L, traceable to the WHO/IFCC reference material, and displays an excellent correlation coefficient of r=0.995 with when compared with other commercially available methods. To accompany this liquid ready-to-use reagent we also offer a dedicated 5 point calibrator with accuracy-based assigned target values (in nmol/l) is available, accurately reflecting the heterogeneity of the apo(a) isoforms.
For more information on this revolutionary assay, visit randox.com/lipoproteina/ or reach out to us at marketing@randox.com.
References
- World Health Organization. Cardiovascular Diseases. World Health Organization. Published June 11, 2021. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). Journal of Lipid Research. 2016;57(8):1339-1359. doi:https://doi.org/10.1194/jlr.r067314
- Zafrir B, Aker A, Saliba W. Extreme lipoprotein(a) in clinical practice: A cross sectional study. International Journal of Cardiology Cardiovascular Risk and Prevention. 2023;16:200173. doi:https://doi.org/10.1016/j.ijcrp.2023.200173
- Pino BD, Gorini F, Gaggini M, Landi P, Pingitore A, Vassalle C. Lipoprotein(a), Cardiovascular Events and Sex Differences: A Single Cardiological Unit Experience. Journal of Clinical Medicine. 2023;12(3):764. doi:https://doi.org/10.3390/jcm12030764
- Stürzebecher PE, Schorr JJ, Klebs SHG, Laufs U. Trends and consequences of lipoprotein(a) testing: Cross-sectional and longitudinal health insurance claims database analyses. Atherosclerosis. 2023;367:24-33. doi:https://doi.org/10.1016/j.atherosclerosis.2023.01.014
- Lampsas S, Xenou M, Oikonomou E, et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules. 2023;28(3):969. doi:https://doi.org/10.3390/molecules28030969
- Vuorio A, Watts GF, Schneider WJ, Tsimikas S, Kovanen PT. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. Journal of Internal Medicine. 2019;287(1):2-18. doi:https://doi.org/10.1111/joim.12981
- Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. European Heart Journal. 2022;43(39):3925-3946. doi:https://doi.org/10.1093/eurheartj/ehac361
- Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. Journal of the American College of Cardiology. 2017;69(6):692-711. doi:https://doi.org/10.1016/j.jacc.2016.11.042
- Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? Journal of Lipid Research. 2016;57(5):745-757. doi:https://doi.org/10.1194/jlr.r060582
- Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. Journal of Lipid Research. 2016;57(7):1111-1125. doi:https://doi.org/10.1194/jlr.r051904
- Svilaas T, Klemsdal TO, Bogsrud MP, et al. High levels of lipoprotein(a) – assessment and treatment. Tidsskrift for Den norske legeforening. Published online January 12, 2023. doi:https://doi.org/10.4045/tidsskr.21.0800
- Wyness SP, Genzen JR. Performance evaluation of five lipoprotein(a) immunoassays on the Roche cobas c501 chemistry analyzer. Practical Laboratory Medicine. 2021;25:e00218. doi:https://doi.org/10.1016/j.plabm.2021.e00218
Randox Health announce partnership with Simplyhealth

Simplyhealth is now offering customers of its Health Plan discounted access to 10 Randox Health home testing kits able to detect common lifestyle related conditions including high cholesterol, vitamin deficiencies or diabetes.
24.4% of the UK population were at risk of at least one underlying health condition in 2019*. Reports from Randox Health home test kits will enable members to act early by suggesting simple lifestyle changes or directing them to a GP for further advice and guidance.
Simplyhealth Health Plan members can purchase discounted tests via the SimplyPlan app or the online Simplyhealth portal, under the SimplyRewards section. Members can choose from 10 Randox Health home test kits that measure up to twenty-four biomarkers related to heart, kidney, liver, iron, diabetes, thyroid, and nutritional health, providing fast, accurate, and convenient insights into their health.
The ten discounted at home test kits are: Vitamin D, Vitamin B12, Heart Health, Thyroid Health, General Health, Male Hormones, Female Hormones, STI, Anti-mullerian hormone (AMH), and Prostate-specific Antigen (PSA). Full details of each test can be found on the Simplyhealth and Randox websites.
David Ferguson, Chief Operations Officer for Randox Health said: “We are pleased to announce this partnership with Simplyhealth, providing their members with discounted Randox Health home testing kits.
“Our range of specialised health packages enable you to take control of your health, using our innovative diagnostic technologies which can give a comprehensive overview of an individual’s health, helping to detect the earliest signs of illness. Together we can work towards preventative healthcare.”
Claudia Nicholls, Chief Customer Officer for Simplyhealth said “A better understanding of the body can empower people to make diet and lifestyle changes that could help prevent future illness. We are passionate about making healthcare more accessible to all and our partnership with Randox Health is just one of the ways we are helping people to take more control of their health and prevent them from becoming ill.
Simplyhealth’s Health Plan for businesses can be tailored to meet the specific needs of any workforce and is available for under £5 per employee, per month, making it more accessible and easier to offer the entire workforce cover. Individuals can access the service as part of Simplyhealth’s 1-2-3 Health Plan from just £20 a month.
World Tuberculosis Day 2024
Tuberculosis in Brief
When we think of Tuberculosis (TB) we tend to think of an old-timey disease. Doc Holliday, the famous gunslinger, died of consumption, the old-world name for TB. As did Fantine from Victor Hugo’s “Les Misérables” and Nicole Kidman’s, Santine, in the 2001 movie, Moulin Rouge! For the videogame fans out there, you might be familiar with Arthur Morgan from Red Dead Redemption 2 who, depending on how you played the game, may have suffered a similar fate. However, this disease is still prevalent around the world today. TB is a bacterial infection caused by Mycobacterium tuberculosis estimated to infect around 10 million people and is responsible for up to 1.5 million deaths each year1.
Originally discovered in 1882, M. tuberculosis is an airborne pathogen which primarily affects the lungs but can also affect other parts of the body2. TB infection exists in 3 states: latent, subclinical, and active. A latent TB infection is asymptomatic and non-transmissible. Subclinical infections are also asymptomatic but transmissible and will produce a positive culture. Finally, active disease is a transmissible state associated with the symptoms of TB2. The World Health Organization (WHO) estimates that around ¼ of the world population is infected with M. tuberculosis3. Up to 15% of those infected with TB will progress to active disease, while those who do not are at a heightened risk of infection throughout the rest of their lives4. Compared with some other bacterial diseases, TB is not particularly infectious. An infected individual is estimated to infect between 3-10 people per year2. However, subclinical TB infections present a challenge in reducing transmission because asymptomatic individuals may unknowingly spread the disease – over 1/3 of TB infections are never formally diagnosed5.
The symptoms of an active TB infection include fever, fatigue, lack of appetite, weight loss, and where the infection effects the lungs, a persistent cough and haemoptysis (coughing up blood). HIV-infection is a major risk factor for TB infection and mortality. Up to 12% of all new cases and 25% of TB deaths occur in HIV-positive persons2. Other risk factors for the development of TB are, malnutrition, poor indoor air quality, Type 2 diabetes, excessive alcohol consumption and smoking1.
TB is present around the world. However, as you might expect from the risk factors, low-to-middle income and developing countries account for a disproportionate number of cases. According to WHO, half of all TB infections are found in 8 countries: Bangladesh, China, India, Indonesia, Nigeria, Pakistan, Philippines, and South Africa.
Without effective treatment, TB will kill and estimated 50% of those infected2. Treatment for TB typically involves first-line antibiotics such as isoniazid, rifampicin, pyrazinamide, and ethambutol, with second-line drugs including fluoroquinolones and injectable aminoglycosides6. Nonetheless, drug-resistant TB accounts for an inordinately large amount of the global AMR burden which can arise from both transmitted and acquired resistance. Resistant M. tuberculosis strains are classified as monoresistant – those resistant to 1 drug; multi-drug resistant (MDR) – those resistant to 2 or more first line treatments, commonly isoniazid and rifampicin; and extensively drug resistant (XDR) – MDR strains which are also resistant to second line therapies like fluoroquinolones and aminoglycosides6.
Global rates of TB have been declining. An estimated 75 million lives have been saved since 20001. Furthermore, between 2015 and 2020, TB incidence fell by 13.5%7. However, the progress made over the last decade has been compromised by the COVID-19 pandemic, illustrated by a, 18% drop in diagnosis between 2019 and 20207. Explanations for this decline include delayed treatment because of lack of access to public transport and healthcare facilities, disruption of laboratory services, a personal desire to avoid the stigma of disease and misdiagnosis due to the similarities in symptoms between TB and COVID-19.
The theme for World Tuberculosis Day 2024 is “Yes, we can end TB!” The WHO have set targets of an 80% decline in new cases and a 90% drop in TB-related deaths by 2030. Screening and preventative treatments are crucial to achieving these goals. Therefore, novel methods of detection which are quick, inexpensive and include drug resistance identification are needed.
Mycobacterium Tuberculosis EQA
It is important for those carrying out TB testing to ensure their instruments and methods are accurate and effective. External Quality Assessment (EQA) programmes are an essential part of this process. QCMD is an independent international EQA organisation primarily focused on molecular infectious diseases to over 2000 participants in over 100 countries.
QCMD offers 2 programmes for those testing for TB through molecular methods: Mycobacterium tuberculosis DNA and Mycobacterium Tuberculosis Drug Resistance.
Mycobacterium tuberculosis DNA EQA Programme
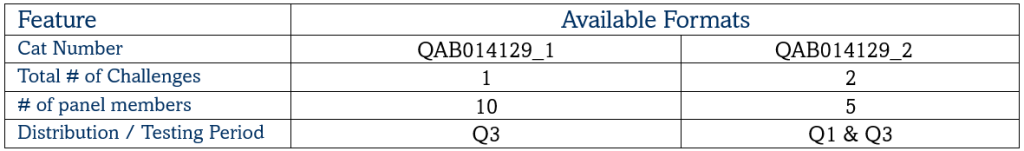
Mycobacterium Tuberculosis Drug Resistance EQA Programme
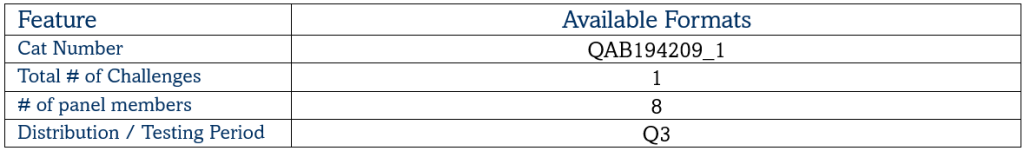
Mycobacterium Tuberculosis Quality Controls
Those conducting research into TB infections and new methods of detection, screening and drug resistance profiling need to be confident that the equipment they are using is up to the task. Qnostics is a leading provider of Quality Control solutions for molecular infectious disease testing. Our range comprises hundreds of characterised viral, bacterial, and fungal targets covering a wide range of diseases.
Q Controls
Our range of positive run, whole pathogen, third party controls are designed to monitor assay performance on a routine basis. As true third-party controls, assay drift is detected, monitored, and managed, helping to ensure accurate and reliable results. The use of third-party controls will also help to support ISO 15189:2012 regulatory requirements.
Mycobacterium tuberculosis (MTB) Q Control 01
Target Pathogen – Mycobacterium tuberculosis (MTB)
Matrix – Synthetic Sputum
Stability – Single use control designed to be used immediately minimising the risk of contamination
Shelf Life – Up to 2 years from date of manufacture
Regulatory Status – Research Use Only

Mycobacterium tuberculosis (MTB) Rifampicin Resistant Q Control
Compatible for use with Cepheid analysers, this whole pathogen positive control is designed to monitor the performance of molecular assays used in the detection of Rifampicin resistant Mycobacterium tuberculosis.
Target Pathogen – Mycobacterium tuberculosis (MTB)
Target Genotype – Rifampicin Resistance
Matrix – Synthetic Sputum
Stability – Single use control designed to be used immediately minimising the risk of contamination
Shelf Life – Up to 2 years from date of manufacture
Regulatory Status – Research Use Only

Mycobacterium tuberculosis (MTB) Evaluation Panel Control
QNOSTICS Evaluation Panels cover a range of genotypes and/or levels, and may be used to evaluate assay characteristics, confirm performance claims, and ultimately ensure the assay is fit for purpose. Evaluation Panels may also be used in the validation of clinical assays and the development of new diagnostic tests.
This dedicated MTB Evaluation Panel comprises 3 targets relating to Mycobacterium tuberculosis for validating a new assay or instrument to ensure that everything is working as expected. High and medium concentrations are provided alongside a negative sample.
Target Pathogens – MTB, M. bovis, Rifampicin (Rif) resistant MTB, Isoniazid (INH) resistant MTB, Negative
Matrix – Synthetic Sputum
Panel Members – 8 (Including a negative)
Stability – Single use. Once thawed, use immediately
Shelf Life – up to 2 years from date of manufacture
Regulatory Status – Research Use Only

If you are interested in any of the TB quality control products shown above, or any other products from our wide catalogue of molecular controls and EQA programmes, get in touch with us today at marketing@randox.com. To learn more, see the links below which will take you to the relevant sites and brochures.
QNOSTICS – www.randox.com/molecular-infectious-disease-controls/
QCMD – https://www.qcmd.org/
References
- World Health Organisation. Tuberculosis. Fact Sheets. Published November 7, 2023. Accessed March 21, 2024. https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers. 2016;2(1):16076. doi:10.1038/nrdp.2016.76
- World Health Organisation. Tuberculosis. https://www.who.int/health-topics/tuberculosis.
- Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of Progression to Active Tuberculosis Following Reinfection With Mycobacterium tuberculosis. Clinical Infectious Diseases. 2012;54(6):784-791. doi:10.1093/cid/cir951
- Adigun R, Singh R. Tuberculosis. StatPearls Publishing; 2024.
- Liebenberg D, Gordhan BG, Kana BD. Drug resistant tuberculosis: Implications for transmission, diagnosis, and disease management. Front Cell Infect Microbiol. 2022;12. doi:10.3389/fcimb.2022.943545
- World Health Organisation. Global Tuberculosis Report 2022.; 2022. Accessed March 21, 2024. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
Patient-Centric, Smart Quality Controls for Immunoassays
In 2022, an updated version of ISO15189 was released, placing an emphasis on risk management with the aim of mitigating risk to patients. This updated document means that rigorous quality control (QC) procedures are more important than ever.
ISO15189:2022 cites the use of third-party controls with commutable matrices manufactured to provide concentrations close to clinical decision limits, among others, as crucial considerations. ISO15189:2022 also highlights the importance of identifying and minimising errors in the pre-analytical process. ‘Load & Go’ or ‘Smart’ quality controls are becoming increasingly popular in laboratories around the world to realise this objective.
Smart controls are designed to optimise laboratory workflows, allowing laboratorians to load the control onto an instrument where it can remain until its expiry date, bringing several advantages to laboratories who run immunoassays.
The first is the minimisation of human error and other pre-analytical errors. As these controls are ready-to-go out of the box, there is no chance of reconstitution errors which can result in deviations from target values and contamination which could lead to problematic cross-reactions. Smart quality controls reduce the risk of stability issues resulting from aliquoting or the repetitive opening of vials, and eliminate the possibility of mislabelled controls, while freeing up more storage space.
Smart controls also offer the possibility of improvements in other areas of the laboratory. The reduction in the preparation required for these controls allows laboratories to use this time improving other elements of their QC practices, such as QC analysis and process improvement. Less steps in the QC process not only means time saved in the process itself, but less paperwork for laboratory staff, further freeing up time for more useful practices.
Immunoassay Smart quality controls provide laboratories with an effective QC solution which aids in the optimisation of workflows and the reduction of test turnaround times and the risk of human error throughout the QC process. However, if considering a Smart quality controls for your laboratory, its important to remember the other factors which make a good QC including matrix, stability, and clinically relevant concentrations.
The New Acusera Smart range has been designed to streamline workflows, minimise human error and reduce the strain on your cold storage. The convenient design means these controls can be loaded directly onto the analyser allowing the automation of the QC process, reducing turnaround times and increasing efficiency.
As well as the Immunoassay control, the Acusera Smart range also includes Clinical Chemistry, Liquid Cardiac and Parathyroid Hormone controls. We offer two options: Acusera SmartScan and Acusera SmartLoad. Take a look at the graphic below for more details.

We will be adding more controls to our Smart range soon. To stay up to date with this and all our other product releases, join our mailing list.
If you’d like some more information on any of the products in the Acusera range, don’t hesitate to get in touch. You can contact us at marketing@randox.com.
New UK-developed test can help predict who will develop type 1 diabetes and unlock treatment

- New drugs are emerging for type 1 diabetes (T1D), but they can only be given before a person has a clinical diagnosis
- Now countries across the world are looking at introducing the first global screening programmes to identify who is at high risk of developing the disease, to prescribe before it is too late
- A new biochip by Randox, developed with the University of Exeter, is the first in the world to use genetics to quickly identify who is at high risk of developing T1D, filtering who should go forward for further testing and accelerating access to treatment where needed.
A new test that uses genetics to help predict who is at high risk of developing type 1 diabetes is now publicly available for the first time, following research that aims to help people across the globe access new drugs that can modify the disease.
The test could help develop new screening programmes for type 1 diabetes, as new drugs emerge which must be prescribed at early stages to be effective. The new test will also help determine type 1 from type 2 diabetes, further improving clinical decision making and treatment.
The test, developed by UK diagnostics company Randox using research from the University of Exeter, could help introduce public health screening in the UK and across the world, supporting those with a high genetic risk of developing type 1 diabetes. Now, the test has received UK regulatory approval – the first such approval issued globally. This means it will be available to consumers in the UK, through Randox Health clinics, and can be ordered online for sample collection at home and returned to Randox’s laboratories for testing.
Type 1 diabetes has a significant inherited risk. The new test is conducted on a Randox biochip which can simultaneously identify up to 10 genetic variants linked to risk for type 1 diabetes. An algorithm is then applied to assess the risk associated with the identified variants for each individual, to calculate a genetic risk score. Previous trials have shown that genetic risk scores are particularly effective in predicting risk for type 1 diabetes. This score will help identify people who don’t have diabetes but are at high risk of developing the disease in the future and can be referred for autoantibody testing to give a definitive diagnosis. The Randox biochip can also be used after diagnosis, to help identify what type of diabetes a person has, which is crucial to ensuring they get the best possible treatment and care.
Identifying those at high risk is particularly topical, as new drugs emerge that can reduce the impact of type 1 diabetes – and they can only be given at the earliest stages, before a clinical diagnosis is given. In November 2022, the US Food and Drug Administration (FDA) approved the use of teplizumab – the first disease-modifying treatment for type 1 diabetes. It can only be prescribed pre diagnosis, yet there is currently no screening programme anywhere in the world to identify early pre-clinical type 1 diabetes. The drug is not yet approved for use in the UK, however, health services globally are now considering how best to introduce public health screening programmes. Diabetes clinician Professor Richard Oram, of the University of Exeter, developed the genetic risk score based on a decade of research, and has worked with Randox on developing the new biochip. He said: “The world is waking up to the value of screening programmes for type 1 diabetes because of new drugs which must be given at the earliest stages of disease. Our new biochip is a pioneering example of how understanding a person’s background genetic risk can help identify those at highest risk, ensuring they have further antibody screening so we can efficiently identify type 1 diabetes early enough for treatment to be effective. The Randox biochip could aid in speeding up decisions
around who should be monitored and tested further, making public health screening cost effective and improving lives by increasing access to treatment.”
Type 1 diabetes affects more than eight million people worldwide, and numbers are projected to rise significantly. The disease causes the body’s own immune system to attack the beta cells which regulate blood sugar. Although the disease is primarily caused by genetics, only around one in ten people with type 1 diabetes have a family member affected, making the other nine in ten difficult to identify. Currently, they are often referred for autoantibody tests when symptoms start to show – but that can be too late to mean they are eligible for treatment.
The new fingernail-sized biochip works by applying DNA extracted from a patient’s blood sample to the biochip surface, upon which copies of the high-risk type 1 diabetes genetic variants are fixed. If a match occurs, the patient’s DNA will bind to the fixed risk variants and emit light. The pattern of positive genetic variants indicates genetic risk and an algorithm is then applied, factoring the significance of each gene variant. The higher the genetic score, the greater the risk that the individual will develop the disease. Those at high risk can then be monitored and put forward for autoantibody screening, while those at low risk need not be screened, which saves money.
Dr Lucy Chambers, Head of Research Communications at Diabetes UK, said: “We’re delighted to see that research supported by Diabetes UK has informed the development of an innovative new tool to find people at high risk of type 1 diabetes. New treatments to prevent or delay type 1 are on the horizon, and their success hinges on establishing effective screening methods to pinpoint those at higher risk. We are continuing to fund research into type 1 screening and are pleased to see new innovations that have the potential to improve lives.”
Hilary Nathan, Director of Policy and Communications at JDRF UK: “For too long, type 1 diabetes has lain silent and undetected to subsequently devastate lives and cause chaos from the first days of diagnosis. This new biochip from Randox and the University of Exeter is exciting, as the test could provide a new way to predict who is at risk from developing type 1. This knowledge then unlocks the opportunity to provide education and intervene at the earliest stages, enabling us to reduce the number of people being diagnosed with diabetic ketoacidosis, which can have traumatic and potentially fatal consequences. We are also on the cusp of a wave of transformative treatments, which can delay the onset of type 1, offering people invaluable years of life free from its burdens.”
Dr Peter FitzGerald MD of Randox said: “We’re delighted to have worked with the University of Exeter on this project to provide a screening tool to assess the genetic risk of type 1 diabetes which, aligned with autoantibody testing, can greatly improve diagnosis, patient care and access to therapeutics. As a result of our regulatory UKCA approval we will, as a world first, be providing this test through our Randox Health clinics, including within certain John Lewis stores, to private individuals in the UK from7th March. We are also releasing the test via a home-based sample self-collection kit. This test is a game-changer in the diagnosis and treatment of type 1 diabetes and we look forward to deploying the test to support public and private healthcare providers globally.”
The benefits of understanding your health status

Randox Health believe in takin a proactive approach to health – making it their mission to deliver accurate and informative preventative health directly to consumers, all the while helping to relieve some of the strain felt by the health service.
More than 4.3 million people in the UK now live with diabetes. Additionally 850,000 people could be living with diabetes who are yet to be diagnoses. (Diabetes UK)
The Office for National Statistics also estimated that one in nine adults – equating to more than five million people – have non-diabetic hyperglycemia, or ‘pre-diabetes’. Making it one of the most common conditions that people live with – most people with diabetes, with the right medication and management, can live completely unencumbered lives and new technologies are making it even simpler. However, if the right care is not provided, diabetes can increase your risk of several serious complications.
Journalist, Matt Rudd, with a group of colleagues from The Times, UK, recently undertook the Randox Health Vital test – which allows you to understand your health baseline by reviewing vital health areas that could increase your risk of heart attack, stroke, and Type 2 diabetes.
Matt described the test as ‘making sense’, “…even though most cases (of type 2 diabetes) are linked to poor diet and obesity those people in the undiagnosed category are likely to be slimmer, younger and in good general health.”
Understanding your health data is vital in helping you to not only feel your best but can also help to prevent illness and reduce your risk of common conditions. The Randox Health Vital health check provides insight into four essential health areas and provides key data empowering you to take control of your health:
- Personal measurements including blood pressure and body composition are measured, If left unmanaged, high blood pressure can increase your risk of heart attack or stroke.
- Heart Health – find out if your levels of both good cholesterol (HDL cholesterol, triglycerides and total cholesterol/ HDL Ratio – higher than normal levels of LDL cholesterol may make you more likely to have heart problems or stroke.)
- Diabetes Health – HbA1c levels are measured to provide an overall picture of average blood sugar levels over a period of weeks/ months. HbA1c levels can be used to indicate prediabetes or diabetes.
- Full blood count.
The easy-to-interpret report from this Health test will provide a breakdown of results, what they mean and next steps.
By finding out if you are at increased risk of conditions such as those that the Vital package tests for, you can choose to make lifestyle changes to help improve your risk of these potentially devastating conditions.
Medical director for Randox Health, Dr. Gary Smyth provided the following comments for the Times article.
“I believe we should be screening more widely. As the adage goes, prevention is better than a cure. We’re not talking about eradicating type 2 diabetes, but we know it’s am entirely preventable disease with the correct lifestyle choices. It’s depression that there isn’t a system to test more people at a younger age.”
To read the full Times article: https://www.thetimes.co.uk/article/35d441ef-49fa-4b1a-978b-13792123cde9?shareToken=6f1dbe1f2bf3e591663f0c240d92c9ae
A Peculiar Problem in Pregnancy and the Placenta
Complications and Diagnosis of Pre-eclampsia
When we consider our most important organ its intuitive to choose the heart, the lungs or even the kidneys. However, there’s another without which none of us would be here to have the discussion. This ephemeral organ provides us with the nutrients necessary for development, removes malevolent agents, provides our initial immunity and much more, before being cast off as we enter the world. We are, of course, talking about the placenta. Indeed, all our organs work together to support life and it’s arbitrary to imbue one with more importance than the others. Nevertheless, as our first organ, the significance of the placenta is irrefutable.
Placental dysfunction, along with several other factors, is known to contribute to the development of pre-eclampsia – a complex, multisystem hypertensive disorder of pregnancy. While the aetiology of pre-eclampsia remains largely unknown, the grave complications associated with it have driven development of novel methods for predicting its onset.
Pre-eclampsia and Epidemiology
Pre-eclampsia is traditionally defined as new onset hypertension and proteinuria in pregnancy1, however, the International Federation of Gynaecology and Obstetrics’ (FIGO) clinical definition describes it as sudden onset hypertension (>20 weeks of gestation) and at least one of the following: proteinuria, maternal organ dysfunction or uteroplacental dysfunction2. It is responsible for an estimated 70’000 maternal deaths, and 500’000 foetal deaths globally3. Pre-eclampsia affects around 4% of pregnancies in the US and is more common in low-to-middle income countries (LMICs), displaying an overall pooled incidence of 13% in a cohort from sub-Saharan Africa4. The risk factors for pre-eclampsia are shown in the graphic below.

Pre-eclampsia is associated with increased morbidity and mortality worldwide. In the US, pre-eclampsia is the foremost cause of maternal death, severe maternal morbidity, maternal intensive care admissions and prematurity5.
Classical classification of pre-eclampsia included early-onset (<34 weeks gestation) and late-onset (>34 weeks gestation). However, this classification lacks clinical utility as it does not accurately illustrate maternal or foetal prognosis. Therefore, the International Society for the study of Hypertension in Pregnancy (ISSHP) and contemporary studies prefer to classify pre-eclampsia as preterm (delivery <37 weeks of gestation), term (delivery ≥37 weeks of gestation) and postpartum pre-eclampsia (after delivery).

Complications
Pre-eclampsia has been associated with acute and chronic complications for both mother and child. Worldwide risk of maternal and foetal morbidity displays adjusted odds ratios of 3.73 and 3.12, respectively (pre-eclampsia vs non pre-eclampsia)6.
Acute Maternal Complications
A range of neurological complications are associated with pre-eclampsia. The most obvious is eclampsia, defined as seizures in pregnant women commonly from 20 weeks of gestation or after birth7. Eclampsia has two proposed mechanisms: abnormal placentation reduces blood supply and causes oxidative stress, leading to endothelial damage; and elevated blood pressure in pre-eclampsia disrupts cerebral vasculature, causing hypoperfusion and damage8. In high-income countries (HICs), most women make a full recovery, however, more severe cases of eclampsia can result in permanent disability or brain damage7.
Stroke is a significant complication of pre-eclampsia, constituting 36% of strokes related to pregnancy9. The hypertension characteristic of pre-eclampsia can weaken the walls of blood vessels causing subarachnoid or intracerebral haemorrhage resulting in haemorrhagic stroke. Ischaemic stroke is also of concern due to blood clotting complications which will be discussed later.
Additonal neurological complications include visual scotoma, cortical blindness, cerebral venous sinus thrombosis, cerebral vasoconstriction syndrome and posterior reversible encephalopathic syndrome (PRES). Notably, the last three in this list frequently manifest postpartum without warning6.
HELLP (Haemolysis, Elevated Liver enzymes and Low Platelets) syndrome is a liver and blood clotting disorder and life-threatening complication of pre-eclampsia. HELLP syndrome most commonly presents immediately postpartum but can manifest any time after 20 weeks of gestation7. Microangiopathy, or small blood vessel disorder, leads to ischaemia and a subsequent increase in oxidative stress and inflammation, causing an increase in liver enzymes and participates in the initiation of HELLP. Thrombocytopenia, or platelet deficiency, is considered a product of platelet depletion resulting from heightened platelet activation triggered by widespread endothelial damage6.
Another blood clotting condition associated with pre-eclampsia is Disseminated intravascular coagulation (DIC)7, described as the dysfunction of the maternal blood clotting system resulting in multiple organ dysfunction syndrome10. DIC can cause excessive bleeding due to lack of clotting proteins, or the formation of clots due to overactive clotting proteins, ultimately causing organ damage10.
As described earlier, proteinuria is included in the diagnostic criteria for pre-eclampsia, suggesting involvement of the kidneys. This is caused by high concentrations of soluble FMS like Tyrosine kinase 1 (sFLT-1), a placental angiogenic factor, which inhibits proteins of the podocyte slit diaphragm6; the machinery involved in preventing the leakage of proteins into the urine11. Reduced levels of Vascular Endothelial Growth Factor (VEGF) and Placental Growth Factor (PlGF) stimulates Endothelin 1 expression6, known to promote podocyte detachment, further contributing to proteinuria12.
Finally, Pulmonary oedema, excessive fluid accumulation in the lungs, is an acute and life-threatening complication associated with pre-eclampsia, the likelihood of which is increased via administration of antihypertensive medications6.
Acute Neonatal Complications
There are several documented complications affecting the baby of a pre-eclamptic mother. Firstly, Intrauterine growth restriction (IUGR) can result in underdevelopment of the foetus because of deficient transfer of oxygen and other nutrients from mother to child13. This can result in low birth weight, particularly when pre-eclampsia occurs prior to 37 weeks of gestation7. In pre-eclampsia with severe symptoms, delivery frequently occurs prematurely, either spontaneously or through induction. Preterm delivery can result in complications such as neonatal respiratory distress syndrome and neonates often require ICU admission7. Additionally, there is increased risk of stillbirth in pre-eclamptic pregnancies with relative risk shown to be 1.45 (95% Cl 1.20-1.76)14. Other complications documented in neonates born through pre-eclamptic pregnancies include neonatal thrombocytopenia, bronchopulmonary dysplasia, and a range of neurodevelopment outcomes15.
Long-term Complications
The only known cure for pre-eclampsia is delivery. However, the complications for both mother and child can last long after even an uncomplicated delivery. After a pre-eclamptic pregnancy, women are increased risk of end stage renal disease (4.7-fold), stroke (4-fold) and vascular dementia (3-fold) later in life5. Women are also at increased risk of other cardiovascular disease (CVD) including chronic hypertension, coronary artery disease, congestive heart failure5, and ischaemic heart disease13. In offspring, IUGR increases the risk of development of hypertension and other CVD13. Finally, offspring have been shown to be at higher risk of increased body mass index, changes in neuroanatomy, reductions in cognitive function, and hormonal abnormalities13.

sFLT-1/PlGF ratio
The pathophysiology of pre-eclampsia is complex and enigmatic. However, placental dysfunction is known to be a factor in pre-eclampsia development. The placental-related angiogenic factors, sFLT-1 (anti-angiogenic) and PlGF (pro-angiogenic), have been implicated in this development. This ratio provides a useful measure of placental dysfunction as a sharp increase in sFLT-1 and decrease in PlGF has been shown approximately 5 weeks before onset of pre-eclampsia16.
Until recently, diagnosis of pre-eclampsia was one of clinical manifestation. However, studies such as PROGNOSIS17 and PROGNOSIS Asia18, along with others19,20, have shown strong utility of this ratio. The PROGNOSIS study showed that a ratio cutoff of ≥38 was useful for ruling out pre-eclampsia within 1 week with a negative predictive value (NPV) of 99.3% or 4 weeks with a positive predictive value (PPV) of 36.7%17. The definitions of pre-eclampsia used by ICCHP and American College of Obstetricians and Gynaecologists (ACOG) have a PPV of around 20%, but when used in combination with the sFLT-1/PlGF ratio, the PPV is enhanced to 65.5% for ruling in pre-eclampsia within 4 weeks.21.
Similar results have been shown in an Asian cohort in the PROGNOSIS Asia Study. Using the same cutoff value, this study reported an NPV of 98.9%18. Furthermore, in a sub analysis of this cohort that looked at Japanese participants, a cutoff of ≥38 displayed an NPV of 100% for ruling out pre-eclampsia within 1 week and a PPV of 32.4% for ruling in within 4 weeks22.
Accurate Identification is Essential
Like all clinical assays, those used to determine the sFLT-1/PlGF ratio are subject to rigorous quality control, essential to ensure accurate results and diagnosis. The complications of pre-eclampsia are severe and often life-threating for both mother and child. Early and accurate identification is imperative for optimal monitoring, management, and timely interventions to reduce the risk of the grave consequences associated with pre-eclampsia.
The utility of the sFLT-1/PlGF ratio has been shown over various large cohorts and provides improved identification when used in combination with established clinical definitions. While the enigma of pre-eclampsia persists, the dedication of the scientific community to unravel its complexities ensures a future where expectant mothers may benefit from more effective and tailored strategies to mitigate the risks associated with this puzzling condition. Continued research endeavours will undoubtedly shape the landscape of maternal-foetal medicine, fostering advancements that hold the promise of improved outcomes for both mothers and their unborn children.
At Randox Quality Control, we’ve introduced our Pre-eclampsia Control to the Acusera IQC range for use with in vitro diagnostic assays for the quantitative determination of PlGF and sFlt-1 in human serum and plasma.
Our true third-party Pre-eclampsia control comes with clinically relevant, assayed target values, is liquid-frozen for user convenience, utilises a human-based, commutable matrix, and has a 30-day open vial stability.
For more information on this, or any of our other controls, browse our brochure, or reach out to us today at marketing@randox.com for more information.
References
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Obstetrics & Gynecology. 2013;122(5):1122-1131. doi:10.1097/01.AOG.0000437382.03963.88
- Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre‐eclampsia: A pragmatic guide for first‐trimester screening and prevention. International Journal of Gynecology & Obstetrics. 2019;145(S1):1-33. doi:10.1002/ijgo.12802
- Karrar SA, Hong PL. Preeclampsia. StatPearls Publishing; 2023.
- Jikamo B, Adefris M, Azale T, Alemu K. Incidence, trends and risk factors of preeclampsia in sub-Saharan Africa: a systematic review and meta-analysis. PAMJ – One Health. 2023;11. doi:10.11604/pamj-oh.2023.11.1.39297
- Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia. Circ Res. 2019;124(7):1094-1112. doi:10.1161/CIRCRESAHA.118.313276
- Dimitriadis E, Rolnik DL, Zhou W, et al. Pre-eclampsia. Nat Rev Dis Primers. 2023;9(1):8. doi:10.1038/s41572-023-00417-6
- NHS. Pre-eclampsia. Health A to Z. Published September 28, 2021. Accessed January 3, 2024. https://www.nhs.uk/conditions/pre-eclampsia/complications/
- Magley M, Hinson MR. Eclampsia. StatPearls Publishing; 2023.
- Crovetto F, Somigliana E, Peguero A, Figueras F. Stroke during pregnancy and pre-eclampsia. Curr Opin Obstet Gynecol. 2013;25(6):425-432. doi:10.1097/GCO.0000000000000024
- Costello RA, Nehring SM. Disseminated Intravascular Coagulation. StatPearls Publishing; 2023.
- Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol. 2020;24(3):193-204. doi:10.1007/s10157-020-01854-3
- Trimarchi H. Mechanisms of Podocyte Detachment, Podocyturia, and Risk of Progression of Glomerulopathies. Kidney Dis (Basel). 2020;6(5):324-329. doi:10.1159/000507997
- Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. American Journal of Physiology-Renal Physiology. 2020;318(6):F1315-F1326. doi:10.1152/ajprenal.00071.2020
- Harmon QE, Huang L, Umbach DM, et al. Risk of Fetal Death With Preeclampsia. Obstetrics & Gynecology. 2015;125(3):628-635. doi:10.1097/AOG.0000000000000696
- Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal Preeclampsia and Neonatal Outcomes. J Pregnancy. 2011;2011:1-7. doi:10.1155/2011/214365
- Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202(2):161.e1-161.e11. doi:10.1016/j.ajog.2009.09.016
- Zeisler H, Llurba E, Chantraine F, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. New England Journal of Medicine. 2016;374(1):13-22. doi:10.1056/NEJMoa1414838
- Bian X, Biswas A, Huang X, et al. Short-Term Prediction of Adverse Outcomes Using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women With Suspected Preeclampsia. Hypertension. 2019;74(1):164-172. doi:10.1161/HYPERTENSIONAHA.119.12760
- Hughes RCE, Phillips I, Florkowski CM, Gullam J. The predictive value of the sFlt‐1/PlGF ratio in suspected preeclampsia in a New Zealand population: A prospective cohort study. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2023;63(1):34-41. doi:10.1111/ajo.13549
- Nikuei P, Rajaei M, Roozbeh N, et al. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth. 2020;20(1):80. doi:10.1186/s12884-020-2744-2
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022;27:42-50. doi:10.1016/j.preghy.2021.12.003
- Ohkuchi A, Saito S, Yamamoto T, et al. Short-term prediction of preeclampsia using the sFlt-1/PlGF ratio: a subanalysis of pregnant Japanese women from the PROGNOSIS Asia study. Hypertension Research. 2021;44(7):813-821. doi:10.1038/s41440-021-00629-x
Meeting Accreditation Guidelines with Acusera 24.7
At Randox Quality Control, we are never finished shouting about how great our interlaboratory comparison and peer group reporting software is. If you’ve had a look yourself, you’ll know exactly why. Acusera 24.7 is full of fetching, interactive charts, and useful, detailed reports, including measurement uncertainty, to help you streamline your QC procedure.
But Acusera 24.7 is so much more than this. Our team are constantly looking for innovative ways to update and improve our live, cloud-based software. Much of this comes from talking to our subscribers and finding out what they want and how they want to do it. Our team also happens to include some serious accreditation enthusiasts. So, we decided to put their passion to work. We’re regularly coming up with new measures to make meeting the guidelines set out by various accreditation bodies, including ISO15189, as simple for you as we can.
In this article, we’ll look at some of the accreditation requirements and the features we’ve included in Acusera 24.7 to simplify the process for you.
QC management tools
Its one thing to look at the features of Acusera 24.7, but what do the various guidelines have to say about QC management tools? Let’s look at some of the major accreditation literature.
ISO15189:2022
The new version of ISO15189 includes updates which aim to place more emphasis on risk management and mitigating risk to the patient. Here’s what the 2022 version has to say about QC management tools:
The Clinical Laboratory Improvement Amendments 1988 (CLIA)
CLIA ’88 regulations are federal standards applicable to all U.S. facilities or sites that test human specimens for health assessment or to diagnose, prevent, or treat disease. These regulations state the following related to QC management:
COLA Accreditation
The Commission on Office Laboratory Accreditation (COLA) is another recognised laboratory accreditation in the U.S. and is a third-party accreditation organisation that ensures laboratories comply with federal regulations, including those set by CLIA. I’m sure you’re catching the trend here:
Meeting accreditation with Acusera 24.7
Acusera 24.7 offers a flexible approach to help laboratories meet all the QC accreditation requirements detailed above, including CLIA, COLA, CAP, and ISO15189.
Our user-friendly, cloud-based software allows users to effortless run statistical analysis including Coefficient of Variation Index (CVI), Standard Deviation Index (SDI), % Bias, Total Error, Sigma Metrics and more! Find out more about how we can aid you in your statistical analysis in our blog, Advanced Statistics with Acusera 24.7.
Acusera 24.7 can also create fully interactive Levey-Jennings charts, and a selection of histograms to provide a wide range of options for the graphical representation of your data. The interactive features of our charts allow you to record events such as lot changes and calibration events directly on to the chart, helping you achieve not just accreditation, but a better understanding of what is going on in your laboratory. You can read more about our charts and the insights you can gain from them at our blog, Charting the course to laboratory excellence.
Acusera 24.7 can also provide you with a variety of reports to help you effortlessly achieve accreditation. From our Statistical Analysis and Exception reports to our Personalised Performance Summary Reports, we can help your laboratory to efficiently identify and document trends or shifts in performance. You can read all about our reports in our blog, Effortless Data Management: Acusera 24.7 Reports.
Measurement Uncertainty
Anyone involved in laboratory quality control will be aware of measurement uncertainty (MU), although that doesn’t mean everyone understands this tricky requirement. MU is defined as a parameter associated with the result of a measurement that characterises the dispersion of values that could reasonably be attributed to the measured quantity.
In other words, MU provides medical laboratories with an estimate of the overall variability in the values they report. The goal of MU is to quantify the doubt or range of possible values around the measurement result, helping to provide an understanding of the reliability and limitations of measurements. This helps ensure measured results are useful and not wildly inaccurate, allows meaningful comparisons with medical decision limits and previous results of the same kind in the same individual and finally, it’s a requirement of ISO15189:2022:
Calculating MU is no simple task and not one that can even be attempted without in depth know-how. These calculations can take a single member of staff 2 full working days to complete. That’s a lot of time away from their normal duties, especially if MU is to be reviewed regularly, as per ISO15189:2022.
Lucky for you, Acusera 24.7 can calculate you MU in seconds, rather than days, and provide you with a report. This report can be shown to your accreditation surveyor, and you can consider the MU box ticked. You can read more about Acusera 24.7 and MU in our Advanced Statistics blog, or in our educational guide How to Measure Uncertainty.
Peer Group Reporting
The peer group reporting features of Acusera 24.7 are much more than just an added extra. Peer group reporting can help speed up the troubleshooting process, allowing you to determine whether an issue you are seeing is unique to you, or evident in the QC data of your peers. It can also provide you with more confidence in assigned target values and help make significant savings by improving your analytical performance, and therefore, your EQA performance.
A peer group reporting programme can also help meet regulatory requirements, like ISO15189:2022:
So, if you’re struggling to find a suitable EQA programme for your analytes, you might just be able to meet your accreditation with the peer group reporting features included in Acusera 24.7.
We’ve only begun to cover the features of this intuitive and efficient software. If you still aren’t convinced that Acusera 24.7 is right for QC data management in your laboratory, reach out to us today at marketing@randox.com. We’re always delighted to hear from you, and we’ll be happy to discuss any of the features of Acusera 24.7, or any reservations you may have.
Our customers can’t believe the gulf in class between Acusera 24.7 and other QC data management programmes.
Don’t get left behind.
Reach out to us today!







