Whole pathogen multiplex controls to support testing for cold, cough and COVID
Randox & Bosch Healthcare – Collaboratively Combating COVID-19
Randox in the media
Latest News
FAQs
Randox response to comments within Sunday Times article 18th October 2020

Randox in the media
Latest News
FAQs
Vivalytic | SARS-CoV-2 Pooling Test

Vivalytic | SARS-CoV-2 Pooling Test
–
Detecting SARS-CoV-2 (COVID-19)
Detecting SARS-CoV-2 (COVID-19) from up to 5 Pooled Samples Simultaneously
Clinical Significance
SARS-CoV-2 Pooling (COVID-19) is a rapid real time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care. This enables the patient to take the recommended safety precautions without delay. The pooling could be done at the level of a ward, medical speciality, social bubble, or group of colleagues. It has potential for use in other settings, such as pre-operative screening, schools and universities, prisons, nursing homes, primary care, and large workplaces. Sample pooling allows more people to be tested quickly using fewer testing resources.
In collaboration with Bosch, we are proud to release not only a rapid testing solution for the detection of SARS-CoV-2 (COVID-19) but an accelerated mass testing solution to effectively and efficiently monitor and detect viral infection from the offset with an aim of minimising the rise in infections globally. The new SARS-CoV-2 pooling test will allow users to test up to 160 samples a day and has sensitivity of 98% and a specificity of 100% – a world’s first!* Highly sensitive assays allow for laboratories to accurately detect low positive samples, enabling for effective identification of positive COVID-19 cases in a timely manner.
* Detailed information on the determination of analytical performance can be found in instructions for use provided with SARS-CoV-2 pooling kit.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab
Sample Volume: 150 μL per-patient sample (If
less than 5 patient samples, supplement the remaining
volume with eNAT solution)
Detection Method: Real-Time PCR
Time to result: 44 minutes
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Related Products
Viral Respiratory Infection Array
SARS-CoV-2 Rapid Test
Vivalytic
Vivalytic Test Menu
EU Traffic Light System: How to get a COVID-19 PCR test before travelling
We Are Randox | How Courtney became a COVID-19 Scientist and Trainer
Vivalytic | Sexually Transmitted Infection Array

Vivalytic | Sexually Transmitted Infection Array
–
1 Sample | 1 Test | 10 Infections
Comprehensive Sexual Health Profile
Clinical Significance
The Sexually Transmitted Infection (STI) array is the broadest multiplex cartridge-based STI test on the market simultaneously detecting 7 bacterial, 2 viral and 1 protozoan infection for a comprehensive sexual health profile.
Globally, each day over 1 million people are exposed to an STI, many of who will not know they have an infection as many STI’s are asymptomatic whilst some individuals will display similar or overlapping symptoms, therefore co-infections may remain undiagnosed. Designed to offer a complete sexual health profile with an aim of prevention and control, the Vivalytic STI array can be used to diagnose existing infections whilst any identifying co-infections.
To find out more about the global economic burden of STIs and the impact of the COVID-19 pandemic on sexual health, download our most recent whitepaper that presents issues such as AMR, the development of super-gonorrhoea, and the challenges faced in a social and public concept due to the COVID-19 pandemic.
Features
Sample Type: Swab or Urine (eNAT, Roche COBAS medium, or PBS)
Sample Volume: 300 μl
Detection Method: Randox Biochip Technology (end-point PCR)
Time to result: 2 hours 20 minutes
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Related Products
Viral Respiratory Infection Array
Vivalytic Test Cartridges
Vivalytic
Vivalytic Test Menu
New 39-minute COVID test available on Randox-Bosch Vivalytic
RESEARCH
BIOPHARMA
CLINICAL LAB
BIOREAGENTS
Vivalytic | Test Cartridges

Vivalytic | Cartridges
–
Powered by Biochip Technology
Simple & Accurate POC Molecular Diagnostics
Vivalytic cartridges are compact, technologically advanced Molecular Diagnostic tests utilising micro-fluidics to enable simple and accurate diagnostic testing. Vivalytic cartridges are powered by a variety of technologies, dependent upon the test application. High-Plex and Low-Plex tests can be analysed on the Vivalytic. High-Plex tests utilise Randox patented Biochip Array Technology, enabling end-point qualitative PCR and providing multiple test results from each sample. Low-Plex tests are based on a variety of detection methods including real-time qualitative PCR and melting curve analysis.
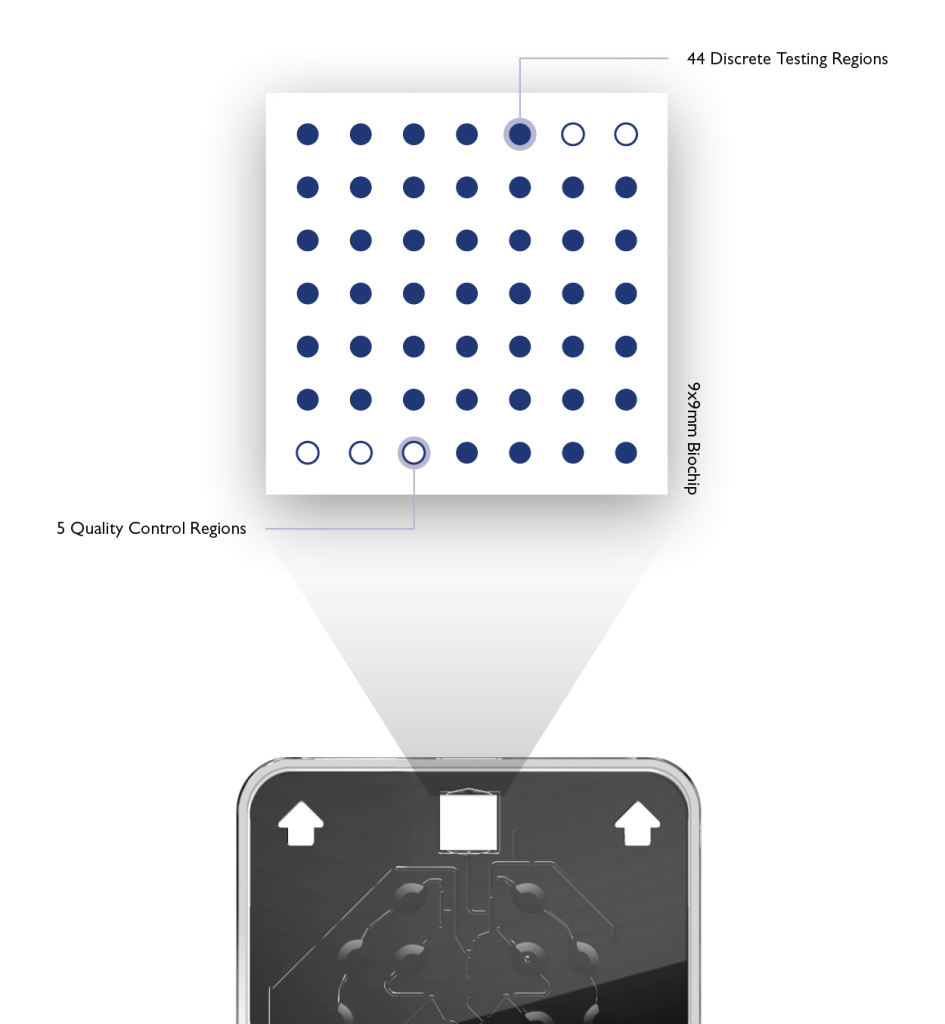
Randox patented Biochip Technology allows simultaneous detection of multiple targets from a single patient sample. The biochip detection system is based on a chemiluminescent signal, this is the emission of light, without heat, as a result of a chemical reaction. Each biochip is prefabricated with spatially discrete testing regions (DTR’s).
Each DTR represents an individual test. Each DTR can be occupied with oligonucleotides specific to a pathogen or target of interest. The High-Plex capabilities of Biochip Technology eliminates the need to run multiple time consuming and sample intensive assays.
An enzyme is used to catalyse the chemical reaction of the biochip which generates the chemiluminescent signal. The light emitted from the chemiluminescent reaction that takes place in each DTR is simultaneously detected and quantified using a Charge – Coupled Device (CCD) Camera. This CCD Camera simultaneously records the light emission from all the DTRs on each biochip. The Vivalytic automatically generates a result report for all targets.
Related Products
SARS-CoV-2 Rapid Test
VRI Array
Vivalytic
Vivalytic Test Menu
Vivalytic | SARS-CoV-2 Rapid 39 Minute Test

Vivalytic | SARS-CoV-2 Rapid 39 Minute Test
–
Detecting SARS-CoV-2 (COVID-19)
Rapidly Detecting SARS-CoV-2 (COVID-19)
Clinical Significance
SARS-CoV-2 (COVID-19) is a rapid real time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care. This enables the patient to take the recommended safety precautions without delay.
In collaboration with Bosch, we are proud to release not only a rapid testing solution for the detection of SARS-CoV-2 (COVID-19) but an accelerated mass testing solution to effectively and efficiently monitor and detect viral infection from the offset with an aim of minimising the rise in infections globally. The new SARS-CoV-2 pooling test will allow users to test up to 160 samples a day and has sensitivity of 98% and a specificity of 100% – a world’s first!*
* Detailed information on the determination of analytical performance can be found in instructions for use provided with SARS-CoV-2 kit.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab (eNAT)
Sample Volume: 300 μl
Detection Method: Real-Time PCR
Time to result: 39 minutes
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Related Products













