World Heart Day – 29th September 2017
World Heart Day – 29th September 2017
World Heart Day – 29th September – Introduction
This year (29th September 2017) join us as we help to raise awareness for World Heart Day! The theme for this year’s World Heart Day is to share the power – and you know what they say… “Knowledge is Power” so throughout this blog we will be providing vital knowledge as well as tips to having a healthy heart!
The heart is a muscular organ that pumps blood around the body and is indeed central to your circulatory system. The system consists of a network of blood vessels, including, veins, arteries and capillaries. These vessels transport blood – as well as carrying oxygen and other important nutrients – to every part of the body. Ensuring a healthy heart is therefore vital.
What is CVD?
When too much pressure is put on our hearts we start to run into some issues – the general term for conditions affecting the heart is Cardiovascular Disease – better known as CVD. The exact cause of CVD is far from clear, with many factors increasing your chances of developing CVD. These risk factors can include, but are not limited to, high blood pressure, smoking, high cholesterol, being overweight or in many cases, can be hereditary.
It is important to note that Cardiovascular Disease is accountable for nearly half of all non-communicable disease (NCD) deaths, therefore making it the number one killer across the globe! Scary thought considering there are a whopping 17.3 million CVD related deaths per year – including stroke and heart disease. Understanding CVD in today’s society is more important than ever before, we need to know the truth about CVD and be able to decipher the facts from the fiction. Below you can see a few examples of common misconceptions regarding CVD and also some that are indeed true.
Only older men can get heart disease/CVD
False
Cardiovascular Disease can develop before birth
True
Exercising won’t help if you’re genetically predisposed to CVD
False
Low and middle-income countries are the most susceptible to CVD
True
It is estimated that by 2030 the number of deaths, due to CVD, will rise to an enormous 23 million globally! However, by raising awareness of the critical numbers and facts we can all help prevent CVD by making small, simple lifestyle changes.
Tips for a Healthy Heart
Using our “art into heart” graphic below, we decided to outline some of our Randox QC top tips for staying healthy! Why not try some of them and feel the effects of having a happy, healthy heart!

This World Heart Day, join us and many more around the world, to raise awareness for this great cause and unite together to “Share the Power”.
Randox launching new product range at leading laboratory medicine event in USA
A group of 30 delegates from global healthcare company Randox Laboratories has this week travelled to the USA, to take part in the world’s largest diagnostics conference – in San Diego, California.
The American Association of Clinical Chemistry (AACC) Annual Meeting and Clinical Lab Expo, known as the leading event for laboratory medicine worldwide, is attended by over 20,000 delegates from across the globe, and offers Randox the opportunity to showcase their capabilities and to network with leading healthcare professionals and key decision makers.
Launching at this year’s event are a number of innovative and exciting new products, including a test for Acute Kidney Injury, a revolutionary diagnostic for small, dense low density lipoprotein (sLDL), a subtype of LDL cholesterol which is a vital risk marker for cardiovascular disease, and the latest in laboratory quality assurance software.
Chief Scientist at Randox Laboratories, John Lamont, who is heading up the delegation to the US, commented;
“Our very significant investment in research and development means that we have more new tests in development than any other healthcare company in the world. Each year at AACC we are able to bring a wealth of exciting new technologies to the American market, for a range of the world’s pressing health issues in need of the most urgent address. We look forward to showcasing our latest innovations at this year’s AACC conference, and to identifying further potential projects.”
Dr. Peter FitzGerald, Founder and Managing Director of Randox Laboratories, commented;
“We appreciate that post-Brexit there will of course be particular business challenges with regards to international business, but at Randox we will remain committed to developing new health diagnostic technologies in the areas where they are needed most, and to expanding the business in our key markets, such as the US.
“The USA is one of our most important markets and we have been exporting our diagnostic products there since the early 1980s. We will continue to nurture our presence there, the expansion of which will be supported by our soon to be opened facility in Kearneysville, West Virginia, which will enable us to strengthen our position in that market.”
AACC runs from the 30th July – 3rd August at the San Diego Convention Centre. Randox can be found at booth #3839.
For further information about Randox at AACC please contact Randox PR on 028 9445 1016 or email RandoxPR@randox.com
Randox increases exports and secures new business in China
Following the launch of its new Chinese market expansion programme in December 2016, global healthcare diagnostics company Randox Laboratories has made significant strides in its business with China, successfully increasing its exports and enabling new business opportunities within the country.
The firm has just returned from a business trip to China to meet new distributors and customers acquired over the last six months, and to train this recently acquired distributor network on Randox’s latest healthcare technologies, with a key focus on the company’s RIQAS programme, the world’s largest External Quality Assessment scheme for laboratories.
Ying Zhu, Sales Manager for Randox Laboratories in China, commented on the success Randox has seen in China over the last six months;
“China is one of our key markets and although our world-leading diagnostic products have been sold there for more than 20 years, we continue to increase our business presence there thanks to increased output from our innovative R&D programmes, and enhanced manufacturing capabilities, including new facilities at the Randox Science Park, our new headquarters in County Antrim. These infrastructural developments have resulted in an increasing range of products which we can now offer to growing and dynamic markets such as China. This increasing range of products coupled with a growing market presence has significantly increased our business penetration and subsequent support to customers.”
Since the launch of the market expansion programme last year Randox has successfully secured distributors across four of the largest provinces of China and has partnered with key hospitals in Guangzhou, Shanghai and Wuhan.
Margaret Fick, RIQAS Scientist added;
“At our most recent training conference in China, in addition to the educational seminars I conducted with my colleagues, we were also delighted to be able to host several of our newly acquired hospital partners, who presented to our audience of current and potential customers on how their laboratories have benefited from our Randox Quality Control products. The use of testimonials from high profile individuals who have experienced the quality, efficiency and reliability of our products is accelerating our growth across China and this is set to continue over the coming months and years.”
For further information about Randox in China please contact Randox PR on 028 9445 1016 or email RandoxPR@randox.com
Committed to meeting customers’ needs
At Randox Quality Control, we strive to meet and exceed customer expectations ensuring high quality products and superior customer service are at the top of our priority list.
How can Randox Quality Control help you?
High Quality QC
The Acusera range of true third party controls boasts an impressive range of benefits ultimately designed to help laboratories reduce costs and time while also ensuring an accurate and reliable test system.
The extended shelf life of our controls allows the same lot of control to be used for a period of up to 2 years keeping costly new lot validation studies to a minimum. We may also be able to sequester lots on your behalf.
The availability of commutable controls designed to react to the test system in the same manner as a patient sample and controls targeted at clinical decision levels will not only help you to meet ISO 15189:2012 requirements but will effectively challenge instrument performance.
Click here to find out more about our QC range.
Customer support
The Randox global support network are on hand with expert advice to ensure timely, accurate and helpful resolution of any issues or queries you may have. The added benefit of quick delivery of product orders further highlights how we work with and for our customers to provide the best service available.
Customer Reviews
Don’t believe us? Read a few of the reviews we have received from laboratories around the world;
“I would like to thank the Randox team for the excellent service when helping to reserve and manage our IQC orders, lot numbers and stock.” – Chief Biomedical Scientist, London, 2017.
Request your free QC consultation by contacting us today! Get in touch and we can arrange for your laboratory to have a consultation with one of our Randox QC specialists. Alternatively, if you would like to leave us a review you can do so by emailing acusera@randox.com.
RIQAS Frequently Asked Questions (FAQ’s)

- How do I register for RIQAS?
Complete the RIQAS method questionnaire and enrolment document for the programmes you wish to participate in. RIQAS will then issue you with a unique laboratory reference number. The enrolment document should be returned to RIQAS before the start of the cycle. These documents can be easily downloaded from the RIQAS website. Simply click on the programme of interest and download the relevant documentation.
- What if my current method is not listed in the method questionnaire or enrolment document?
Use the method questionnaire to help you complete the registration of methods section on the enrolment document. If a code is not available for your method/assay please state the details of your method clearly in the appropriate section at the end of the enrolment document.
- How do I enter my EQA results?
Participants may conveniently enter their results online via RIQAS.Net. Alternatively results can be entered via the manual return sheet and submitted by fax or post before the final submission deadline.
- How do I know when to submit my EQA results?
Each RIQAS pack will contain a multi-lingual product insert containing instructions for use. The product insert also highlights the recommended date of analysis and more importantly the final date by which the results must reach Randox. The final date for submission of results can also be found on the RIQAS calendar. All results should reach RIQAS before 5pm GMT on the final submission date.
- How and when are RIQAS reports issued?
For most programmes reports are available within 72 hours of the final submission date (for RIQAS Serology Programmes, reports are sent via email within 7-10 days of the final submission date). The reports may be accessed online via RIQAS.Net or alternatively may be sent by email or post. Individual reports may be emailed to up to three addresses.
- Can I register multiple instruments for a single EQA programme?
Yes, laboratories can register up to five instruments per programme at no extra cost. Individual reports for each instrument plus a unique multi-instrument report are provided. The multi-instrument report allows for comparative performance assessment of each instrument. Additional sample packs may be ordered as required.
- What is the summary CSV file?
Laboratories can register to receive a CSV file containing a summary of their report statistics, acceptable limits and performance indicators for every sample. The file mirrors the information found on the quantitative report summary page but will also include the calculated SD and SDPA. If you wish to receive a summary CSV file, please indicate this by ticking the box on the enrolment document and include the email addresses to which the reports should be sent.
- Is RIQAS accredited to ISO/IEC 17043:2010?
Yes, in 2012 RIQAS celebrated gaining accreditation to ISO/IEC 17043:2010. This standard outlines general requirements for proficiency testing and demonstrates our commitment to quality whilst providing both participants and accrediting bodies with confidence in the schemes operation. Our accreditation to ISO/IEC 17043:2010 highlights the superior quality and excellence of RIQAS. **Please be aware that not all RIQAS programmes are accredited. Programmes marked with a “+” highlight the programmes not accredited.**
9. What if I don’t need all the parameters in a particular EQA programme?
Reduced parameter options are available for selected EQA programmes offering greater flexibility, whilst ensuring suitability for laboratories of all sizes and budgets.
10. How does the group reporting facility work?
The Group Reporting facility enables group co-ordinators to monitor the performance of satellite sites. Each individual laboratory in the group will receive an individual report, the group supervisor will receive a unique instrument group report comparing each laboratory’s performance within the group.
11. Will I receive a certification of participation?
Yes, RIQAS provides certificates as proof of EQA participation and performance for laboratory accreditation purposes. A complimentary certificate of participation for each RIQAS programme is available to participants at the end of each cycle, provided at least 50% of results have been returned. A certificate of performance is also supplied with the end-of-cycle report. Certificates will specify the cycle number, programme name and the LABORATORY / HOSPITAL NAME specified in the enrolment document.
12. Can you offer technical support and advice?
Unrivalled technical support is available through our team of RIQAS scientists and experts who are on hand to offer advice and to help you troubleshoot technical issues relating to our RIQAS programmes.
13. I have found a transcription error on my report. Can I submit the revised result?
Participants are permitted to submit corrected results up to 4 weeks after the final date of the sample. Although a new report will not be issued, results can be viewed on the charts of subsequent reports, showing “C” in place of the sample number. If a result is corrected and resubmitted to RIQAS before the final date for the current sample, it will be entered as a current result.
14. How do I notify a change of method if the cycle is already underway?
It is possible to change your units, method, instrument or reagent classification during a cycle. For participants using RIQAS.Net changes can be made in the method changes section of the data entry menu. Each RIQAS return sheet also has a section for method changes.
15. How do I add extra parameters to my registration?
Extra parameters can be added to a registration via RIQAS.Net using the method changes section on the data entry menu. A list of your registered laboratory reference numbers will appear on screen. Select the laboratory reference number for which you would like to add the assay details and select ‘Add Parameter’. A list of parameters you are not registered for will appear. Select the parameters you wish to add and complete the assay details. Parameters cannot be deleted on RIQAS.Net. If you wish to delete a parameter please contact RIQAS directly on mail@riqas.com.
QC Material Stability – Dig a Little Deeper
QC Material Stability
Stability has a number of different definitions, however, the most relevant to clinical diagnostics, and indeed quality control sera, is the “resistance to chemical change or physical disintegration”. Much like a chain, your quality control system is only as strong as its weakest link, or in this case analyte.
Whilst we appear to be stating the obvious here, this might not be as straightforward as it first appears. The product literature you peruse will help you decide what control best suits your needs, whilst many companies will state their control stability in the literature there are some instances where all may not be as it first appears. It is also important to note that some manufacturers may not make stability claims for some of the analytes listed in their control material. In such instances, you are required to validate these in-house, taking up precious time and resources.
Dig a Little Deeper
Whilst we understand that some analytes do have limitations due to their inherent nature, misleading analyte claims can cost the laboratory both time and money. In a recent survey conducted by Randox, 65.5% of respondents indicated that they felt stability was a ‘Very Important’ QC feature. As such it’s important that you look beyond the sales literature when it comes to control stability. Look out for exceptions in the small print of the control kit inserts. For example, if a control has a stability claim of 7 days at 2-8oC and a routine analyte like Cholesterol has a stability claim of just 2 days at 2-8oC then the true stability of the control is only 2 days. In such instances, there is a lot of potential for waste, as laboratories will be required to prepare a new vial of QC material every 2 days leading to increased costs and time. However, if you dig a little deeper into the controls and always read the small print, you could avoid such issues.
How can Randox Acusera benefit you?
For more than 30 years Randox has been shaping the future of clinical diagnostics with our pioneering high quality, cost effective laboratory solutions. Quality Control is our passion, we believe in producing high-quality material that can help streamline procedures, whilst saving money for laboratories of all sizes and budgets. We pride ourselves in not misleading our customers with false stability claims for our controls. With controls such as our Liquid Cardiac and Specific Proteins Controls, you could benefit from a 30-day open vial stability for all analytes, without exception.
By employing our Randox Acusera quality control materials you could benefit from;
Commutable controls, ensuring a matrix that reacts to the test system in the same manner as a patient sample, enabling an accurate and reliable assessment of instrument performance.
Accurate target values that won’t shift throughout the shelf life of the controls, eliminating the need to spend valuable time and money assigning values in-house.
Consolidation of test menu with controls comprising up to 100 analytes, reducing preparation time and storage space required.
Analytes present at clinically relevant levels ensuring accurate test system performance across the clinical range, maximising laboratory efficiency by eliminating the need to purchase additional high or low-level controls at extra expense.
True third party controls designed to provide an unbiased assessment of performance, our Acusera controls have not been manufactured in line with or optimised for use with any particular reagent, method or instrument.
For more information on any of our products, or to request a consultation from one of our QC Consultants, contact us via acusera@randox.com.
Clin Lab 2017

CLIN LAB 2017
Randox Laboratories will be attending Clin Lab from 6th – 8th April 2017. We look forward to meeting you at Pragati Maidan Exhibition Center, India at Stand No. 9C08, Hall 9.
Randox Laboratories is a global leader in healthcare diagnostics. We are dedicated to developing solutions that not only meet your needs, but that are of the highest quality, the most reliable and the most cost-effective.
JOIN US FOR LIVE DEMONSTRATIONS
Drop by our stand to receive a live demonstration of our latest products including Acusera 24.7, RX imola and RX daytona+. These will take place at Stand No. 9C08, Hall 9 from Thursday – Saturday.
Acusera 24.7
Reduce time spent troubleshooting and analysing QC data with unique access to instantly updated peer group statistics and automatic calculation of Measurement Uncertainty, Sigma Metrics and Total Error all on one centralised platform.
RX series
The RX Series is globally renowned for quality and reliability, teamed with one of the most extensive dedicated clinical chemistry test menus on the market you can benefit from real cost savings through the consolidation of routine and specialised tests on a single platform.
ALSO SHOWCASING
RX series
The RX series of clinical chemistry analysers combines robust hardware and intuitive software ensuring unrivalled precision and accuracy for results you can trust.
Reagents
Committed to advancing biochemistry testing for laboratories. We offer a range of high quality reagents that cover routine and novel markers with applications for use on a wide range of clinical chemistry analysers.
Quality Control
Delivering accurate results, Randox QC provides the complete laboratory package combining true third party controls, calibration verification material, interlaboratory data management software and the world’s largest international EQA scheme – RIQAS.
What is Measurement of Uncertainty?
Measurement Uncertainty (MU) relates to the margin of doubt that exists for the result of any measurement, as well as how significant the doubt is. For example, a piece of string may measure 20 cm plus or minus 1 cm, at the 95% confidence level. As a result, this could be written: 20 cm ±1 cm, with a confidence of 95%. Therefore, we are 95% sure that the piece of string is between 19 cm and 21 cm long.
Standards such as ISO 15189 require that the laboratory must determine uncertainty for each test. However, they have not specified how this should be done.
How do we calculate Measurement Uncertainty using QC data?
Employing your QC data to calculate uncertainty makes several assumptions; your test system is under control, the patient samples are treated in the same manner as your controls and gross outliers have been removed. If you choose to use your QC data to calculate this you should ensure that you use a commutable control with a matrix similar to that of a patient sample, with analytes present at clinically relevant levels
To calculate MU, labs must look at the intra-assay precision and inter-assay precision of their test.
Intra-assay precision: Sometimes known as ‘within run’ precision, is where 20 or more replicates of the same sample are run at the same time, under the same conditions (calculated from a single experiment). Intra-assay precision helps to assess systematic uncertainties
Inter-assay precision: Sometimes known as ‘between run’ precision, is where 20 or more replicates are run at different times – e.g. 1 replicate every day for 20 days (can be calculated from routine IQC data). Inter-assay precision can help identify random uncertainties within the test system.
*The Australian Association of Clinical Biochemists (AACB) recommends that at least 6 months’ worth of QC data are used when calculating the inter-assay precision1.
Once the data is collected, you must calculate the standard error of the mean (SEM) of the intra-assay precision (A) and the SD of the inter-assay precision (B) in order to measure the uncertainty (u). Once A and B have been calculated, they need to be squared, added together and the square root of the sum found:
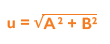
As uncertainty is calculated as SD and 1SD is equal to 68% confidence on a standard Gaussian curve, we can conclude that if we multiply using a coverage factor of 2, we can attain 2SD confidence of 95%. This is known as the Expanded Uncertainty (U):
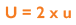
What is the Advantage of Measurement Uncertainty for a lab?
Labs need to carry out MU as it is a requirement of ISO 15189. It states: “The laboratory shall determine measurement uncertainty for each measurement procedure, in the examination phases used to report measured quantity values on patients’ samples. The laboratory shall define the performance requirements for the measurement uncertainty of each measurement procedure and regularly review estimates of measurement uncertainty”.
MU also helps determine whether the difference between two results is negligible due to uncertainty or significant due to a genuine change in condition of the patient; giving labs a greater confidence in reported results.
How can Randox help?
Our new Acusera 24.7 Live Online software provides automatic calculation of MU, saving valuable time and helping labs meet ISO 15189 requirements with ease.
Contact marketing@randox.com to find out how your lab can benefit from Acusera 24.7 Live Online
Take steps to prevent incorrect patient results by making one simple change
According to the NHS Litigation Authority; in 2015 within the UK alone, £193,680,744.30 was spent on ‘wrong diagnosis’ or ‘failed/delayed diagnosis’ causing huge financial strain and impact on labs.
With approximately 75% of clinical decisions and diagnosis based on laboratory test results. The only way to guarantee a high degree of accuracy is to implement a good Quality Control plan. The importance of this is recognised globally, several bodies exist internationally including ISO (International organisation for standardisation) who have developed a set of guidelines and quality systems to ensure the reliability of laboratory test results.
So what can you do to improve accuracy and reliability?
Choose a third party QC
ISO 151589:2012 Section 5.6.2.2 states that “the use of third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer”.
First Party Controls are those manufactured by the instrument/reagent manufacturer. These controls are optimised specifically for use with the manufacturers test system and therefore will mask a multitude of weaknesses. First Party Controls tend to result in perceived accuracy and a biased assessment of performance.
Third Party Controls on the other hand are designed to be completely independent and are not optimised for use with a specific test or system. Leading manufacturers of third party controls will assign target values based on data collected from thousands of independent laboratories, ensuring the availability of statistically robust multi-method, multi-analyser data. Therefore laboratories using Third Party Controls can be assured of unbiased error detection across multiple platforms.
Randox Acusera is a world leading manufacturer of true third party controls providing a cost effective, high quality solution for any laboratory-regardless of size or budget.
Look out for QC samples with clinically relevant concentrations
ISO 15189:2012 states that ‘The laboratory should choose concentrations of control materials wherever possible, especially at or near clinical decision values, which ensure the validity of decisions made’.
It is important to assess the full clinical range of an assay i.e. the range between the lowest and highest results which can be reliably reported. In order to make sure a laboratory instrument is performing accurately across the full clinical range and in particular at the medical decision level, QC materials that cover low, normal and elevated concentrations should be used.
Due to the superior manufacturing process used by Randox, QC target values consistently cover the MDL of tests. By ensuring the controls in use cover clinical decision levels laboratories can be confident of the reliability and accuracy of the patient results they release.
Opt for a commutable control material
A good QC material has many essential properties but above all, controls must perform consistently and reflect the performance of patient samples – if a control meets these requirements then we can say it is commutable. Having a commutable control would aid in the prevention of incorrect patient results because they replicate the performance of a patient sample and react to the test system in a similar manner. Use of a commutable control will also reduce costly shifts in QC target values when reagent batch is changed.
At Randox we take quality seriously, that’s why all QC products are manufactured to the highest possible standard, delivering controls of unrivalled quality. Designed to be commutable, the Acusera range will ensure accurate and reliable instrument performance while simultaneously helping laboratories to meet ISO 15189:2012 requirements. A good QC process will include the use of Third Party Controls, Clinically Relevant Concentrations and controls which can be described as commutable. By employing Quality Control’s that encompass these traits, a laboratory professional can be certain that they have taken the necessary steps to decrease incorrect results and therefore potential misdiagnosis.
Acusera 24•7 Frequently Asked Questions (FAQ’s)

Is it possible to compare multiple instruments on a single Levey-Jennings Chart?
With Acusera 24•7 users can combine multiple instruments, as well as analytes and QC lots on a single chart. This will enable comparative performance assessment and facilitate immediate visualisation of any ongoing or emerging trends. It may also be useful for troubleshooting out of control QC events.
Is it possible to compare different levels of control on a single Levey-Jennings Chart for identification of concentration related bias?
Yes data for multiple QC lots can be displayed on a single, convenient Levey-Jennings chart allowing any concentration related problems to be identified quickly.
How often is peer group data updated?
With Acusera 24•7, peer group data is uniquely updated live in real-time. The instant nature of the peer data will help reduce time and money spent troubleshooting, re-running QC samples and performing any instrument maintenance. With real-time peer group data you can compare to other laboratories around the globe using the same lot of QC material and identify if there are any issues and whether they are unique to your lab or a widespread issue.
Is there a limit to the number of users?
There is no limit to the number of concurrent users – you can have as many as you want. There are 5 different levels of user access – Admin, Group Co-Ordinator, Manager, User and Technical Support. It is worth noting that User access can be customised per user to ensure access to only the required functionality
How can the software help me to meet ISO 15189:2012 requirements?
Unique to Acusera 24.7 , our software will automatically calculate Measurement Uncertainty (UM) and provide your laboratory with a printable report that can be used to help meet ISO 15189:2012 accreditation requirements. In addition to this the software can help to prevent the release of patient results in the event of a QC failure for example when the Quality Control rules are violated.
Is it possible to use RiliBAK as my allowable limits?
QC data can indeed be rejected or alerted based on RiliBAK guidelines. Other options are also available, including, CLIA, Biological Variation, RIQAS TDPA and user defined performance limits.
Is Acusera 24.7 secure?
To authenticate users, a number of security measures are used, including; participant number, username and a password combination (for individual role-based accounts). Password complexity standards are enforced on user account setup. CAPTCHA is enforced after several failed login attempts to prevent or guard against automated attacks. HTTPS and X509 certificate authentication is in place meeting industry security standards.
I have never used interlaboratory software before, is there training provided with my purchase of 247?
There are a number of different options available in terms of training. The easiest, most convenient and accessible form of training is through the use of our walk through demo that has voice-over and text so everyone can follow. With our software comes a user guide, this is a walk through that laboratories can use to guide them through the usage of the software. Additionally, there is also the possibility of a live demo from the sales consultant in your area who will be able to run through the software with you.
Can I create unique login credentials for each user?
Yes, each user will have their own personal log-in. If a lower level user, e.g. user, technical support or manager forgets their password they can have it reset by the administrator. However, if a group co-ordinator or admin forgets their password they should contact Randox directly who will be able to reset their credentials and resend to the administrator’s email address.
I currently use non-Randox Quality Controls, does this affect the ability of the software?
Not at all, our software is so flexible that it can be used with any other manufacturers Quality Control material. However, you will only have access to the internal functions of the software and will not have access to peer group statistics. For this reason we recommend the software is used alongside our Acusera true third party Quality Control solutions.
Is Acusera 24.7 the only software option available?
Yes this is the only option available – Unlike other manufacturers there is no need for any additional software packages or options. All functionality is available in the one software package.
My computer is very old, do I need a new, modern and up-to-date operating system to run the software?
Not necessarily. As long as you have stable access to the internet you can access Acusera 24.7 as it is a cloud based software.
My patient data is confidential. Will the software need access to this data?
Acusera 24.7 will not require access to your patient data. This is important for a laboratory but less for the software. Acusera 24.7 will only need access to the results of your Quality Control to ensure that your instrument/s are performing to standard and therefore ensuring that your patient results will be reliable and accurate.
I have forgotten my username and password – what do I do now?
If an individual with user level or manager level access forgets their username and password, they should contact the laboratory administrator. If an administrator or group co-ordinator forgets their username or password they should contact Randox who will verify the administrator and send new login details for the account.
Is Acusera 24.7 Connect required to import QC data?
Acusera 24.7 Connect is only required if you wish to import QC data automatically. Data can also be entered manually using the data entry screen or in a semi-automated manner using the EDI function.
I need to renew my license, is this done automatically?
If your licence has expired and you would like to renew you should get in touch with your local Sales Consultant.
My internet connection isn’t great. Will this affect the running of the software? What happens if the signal drops when entering results?
If connection is lost from the laboratory’s side, all data will be transferred to the web and once reconnected, the previous session will also be remembered. Emergency power generators and fall over servers are in place to ensure 99.8% uptime is guaranteed.
Are there any additional software requirements?
You must have access to a Java applet. This software is available as standard on almost all modern computers, laptops and notebooks.

























