Randox – Diabetes

During December, we aim to highlight how the Randox product portfolio can be used for accurate diagnosis and monitoring of diabetes, with a focus on the Randox Reagents diabetes panel which offers a total of 12 assays for accurate and reliable diabetes testing.
Diabetes is one of the leading causes of death worldwide and it is estimated by WHO (World Health Organisation) that 2.2 million additional deaths are being caused by the condition each year. The number of people with the condition has being growing rapidly in the last 30 years, the International Diabetes Federation predicts that approximately 438 million people will have diabetes by 2030. Early diagnosis and constant monitoring of diabetes is essential in order to manage the condition, as diabetes can lead to other health problems such as heart disease, kidney damage or failure, nerve damage and even blindness.
Randox knows that this condition cannot be ignored as each year it is increasingly becoming a burden on the health service. Randox Reagents are committed to advancing diabetes related testing and offer an extensive range of high quality reagents: from diabetes diagnosis, to the monitoring of diabetes-related complications, the Randox Reagents diabetes testing panel covers the full spectrum of clinical testing requirements.
Reagents Diabetes Testing Assays

To aid with the growing concern of diabetes, Randox Reagents offer a comprehensive range of 12 assays within their diabetes testing panel including assays for the diagnosis and monitoring of diabetes which includes fructosamine, glucose and HbA1c and also those which monitor diabetes-related complications such as adiponectin, cystatin c, microalbumin and NEFA. The Randox diabetes reagents offer a range liquid ready-to-use and lyophilised formats for increased efficiency, applications are also available for a wide range of biochemistry analysers.
RX series Direct HbA1c Testing Capabilities
Renowned for quality and reliability the RX series range of clinical chemistry analysers boasts a world leading test menu with an extensive range of high performing and unique assays available. In addition to NEFA, D-3-Hydroxybutyrate (Ranbut) and Fructosamine the RX series welcomes Direct HbA1c testing on the RX Daytona +, RX imola and RX modena. The latex enhanced immunoturbidimetric method improves laboratory performance and time, highly improving accuracy and precision by revolutionising your diabetes testing capabilities.
Quality Control

Designed for use in the Quality Control of both HbA1c and Total Haemoglobin assays, our Acusera HbA1c controls are an ideal match for laboratories running these parameters and POCT testing. Available in liquid ready-to-use or lyophilised formats, these controls offer attractive stability and flexibility for labs and healthcare practices of any size. Manufactured using human whole blood which ensures commutability, our controls directly mimic the performance of real patient samples helping deliver reliable results.
RIQAS Glycated Haemoglobin Programme
Designed to monitor the performance of HbA1c, our RIQAS glycated haemoglobin EQA program is suitable for both qualitative and quantitative methods of analysis. As the largest EQA scheme in the world, access to large peer groups is guaranteed. Additional benefits include; monthly analysis, user-friendly reports allowing at-a-glance performance assessment, ability to register up to five instruments per programme and cost savings via our unrivalled consolidation.
Randox – Drugs of Abuse

Throughout November our aim is to highlight how the Randox product range can be utilised to allow for the most accurate analysis of drugs of abuse, with a particular focus placed with the Evidence MultiSTAT, a fast, fully automated and versatile immunoanalyser that enables on-site detection of up to 21 drugs of abuse from a single sample of oral fluid, urine or blood.
The abuse of drugs is a growing problem worldwide and represents a significant burden to healthcare systems as well as creating problems in an individual’s lifestyle. It has been estimated by the WHO (World Health Organisation) that 31 million people globally suffer from drug use disorders and 3.3 million deaths each year are linked to the abuse of both drugs and alcohol.
Randox have reacted to this growing concern and are now a world leader in the drugs of abuse testing field. Our product range currently comprises classical, prescription and synthetic drugs.
The Evidence MultiSTAT offers a simple drug screening solution to those who have little or no knowledge of laboratory procedures. As an extremely versatile, desktop analyser it is ideally suited to a variety of settings including both the clinical laboratory and the emergency room.
Evidence MultiSTAT

The Evidence MultiSTAT utilises Randox’s revolutionary Biochip Array Technology to enable on-site simultaneous detection of up to 21 classical, prescription and synthetic drugs from a single patient sample. The MultiSTAT has the ability to run samples of whole blood, urine and oral fluid with a 2 step process from sample entry to results with the first result coming through after 17 minutes. The MultiSTAT offers an extensive test menu and requires no laboratory experience with it’s simple process of providing rapid screening and reliable results.
RX series Toxicology Testing

Renowned for quality and reliability the RX series range of clinical chemistry analysers boasts a world leading test menu comprising both therapeutic drugs and drugs of abuse. Our toxicology range comprises amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine metabolite, ecstasy, EDDP, ethanol, methadone and opiates. The RX series range of clinical chemistry analysers ensures a high degree of accuracy with a wide range of testing throughputs to suit all laboratories big or small.
Reagents Drugs of Abuse Assays

In order to assist in dealing with the ongoing burden of substance abuse, Randox Reagents offer a comprehensive range of 10 assays to test for some of the top commonly abused substances including, alcohol, methamphetamine’s, cocaine, methadone, cannabis, benzodiazepines, barbiturates, EDDP, ecstasy and opiates. The Randox drugs of abuse reagents are liquid ready-to-use for increased efficiency with applications available for over 30 different analysers.
RIQAS Urine Toxicology Programme

Designed to monitor the performance of 20 drugs of abuse tests in urine, our RIQAS urine toxicology EQA program is suitable for both qualitative and quantitative methods of analysis. As the largest EQA scheme in the world, access to large peer groups is guaranteed. Additional benefits include; monthly analysis, user-friendly reports allowing at-a-glance performance assessment, ability to register up to five instruments per programme and cost savings via our unrivalled consolidation.
Randox – Cholesterol

Throughout the month of October our aim is to highlight how the Randox clinical product range can be used to allow for the most accurate diagnosis and monitoring of cholesterol. As October is national cholesterol month, we want to raise awareness of the many dangers associated with cholesterol to better assess the risk of related complications.
Cholesterol plays a major role in atherosclerosis, the leading cause of cardiovascular disease (CVD). CVD is the leading global cause of death; accounting for over 17.9 million deaths in 2016. This figure is expected to grow to more than 23.6 million by 2030 despite the treatment of individuals with high LDL Cholesterol with statins. These statistics emphasise the urgent need for additional risk factors to be considered beyond traditional LDL Cholesterol measurement.
As a world leader in the field of cardiac risk and lipid testing, Randox are tackling the need for better cholesterol testing with our wide range of niche and high-performance assays including sdLDL Cholesterol, Lipoprotein (a) and HDL3 Cholesterol. In fact Randox is responsible for more than 15% of all cholesterol tests carried out across the globe. Read on to find out more about our complete range of clinical solutions.
Reagents

Typically HDL Cholesterol, LDL Cholesterol and Triglycerides are measured to asses coronary heart disease (CAD) risk however these current methods only detect around 20% of all CAD patients. Recognising the need for more comprehensive risk assessment, the Randox range comprises sdLDL Cholesterol (sdLDL-C), HDL3 Cholesterol and Lipoprotein (a) amongst others. sdLDL-C is a vital risk marker for Myocardial Infarction (MI), research indicates that individuals with a predominance of sdLDL-C have 3-fold increased risk of MI. HDL3 Cholesterol on the other hand is widely accepted to have an inverse relationship with CVD risk, individuals with higher HDL3 Cholesterol levels are therefore at lower risk of developing CVD. Finally Lipoprotein (a) is an independent genetic risk factor for CVD with elevated levels often seen in patients with an otherwise normal lipid profile. Our complete range comprises 113 third party diagnostic reagents, applications are available for a variety of biochemistry analysers. To find out more about our cardiac risk assays click here.
RX series

Renowned for quality and reliability the RX series range of clinical chemistry analysers boasts a world leading test menu comprising both routine and novel lipid tests for accurate & reliable CVD risk assessment. In addition to novel assays such HDL3 Cholesterol, sdLDL Cholesterol, Lipoprotein (a) and Apolipoproteins, the RX series offers superior methodology for the accurate measurement of routine assays including HDL Cholesterol and LDL Cholesterol. Our superior direct clearance method for HDL cholesterol ensures a high degree of accuracy even with abnormal samples containing elevated levels of triglycerides. Similarly no sample pre-treatment is required with our direct clearance method for LDL Cholesterol resulting in less variability and excellent correlation to ultracentrifugation methods. To view the complete RX series test menu and discover more on our testing solutions click here.
Internal Quality Control

The Acusera Lipid controls offer testing for the complete lipid profile, including HDL, LDL and Total Cholesterol. Available in either liquid ready-to-use or lyophilised formats, our controls are ideal for laboratories of any size with flexible options available for each control. Our lipid controls are manufactured from 100% human serum, ensuring commutability, and therefore mimic the performance of a patient sample.
External Quality Assessment

Designed to monitor the performance of up to 7 lipid parameters, the RIQAS Lipid EQA programme is manufactured from 100% human serum ensuring commutability. With access to large peer groups and user-friendly reports supplied, RIQAS is the EQA scheme for your laboratory. Frequent reporting and flexible programme options are only two of many benefits associated with the RIQAS Lipid Programme.
Randox – Infections

Randox are dedicated to improving health worldwide. Throughout the month of September we hope to highlight how the Randox clinical product range can assess the impact of infection through swift diagnosis, allowing the necessary steps to be taken in order to improve an individual’s health. Infection is the infiltration of an organism’s bodily tissues by disease-causing agents, their multiplication, and the reaction of host tissues to the infectious agents and the toxins they produce. The Randox portfolio comprises a wide range of products to assess the impact of infection. The RX series dedicated testing panel comprises of IgA, hsCRP and ASO which are also available for third-party use. The extensive QC range caters for assessment of infectious disease testing in both liquid & lyophilised formats.
Reagents

The Randox range of third-party reagents enables the clinical analysis of 113 different analytes with comprehensive range measurements and excellent correlations to reference methods. Applications are available detailing instrument-specific settings for a variety of biochemistry analysers. Randox offer several assays for the detection of infection.
IgG (the most abundant antibody) and IgM (the first antibody made in response to infection) can be used in the diagnosis of Dengue Fever. More than 40% of the global population, in more than 100 countries are at risk of the Dengue Virus (WHO). IgA is an antibody that lines the mucous membranes lining the mouth, airways, and digestive tract. A deficiency in IgA is common in patients with bronchitis, conjunctivitis and otitis media. Other Randox assays that may be used detect differing infections include: albumin, ferritin, alpha-1-antitrypsin (AAT), complement C3, complement C4, haptoglobin, CRP, alpha-1-acid glycoprotein (AGP) and anti-streptolysin (ASO).
RX series

The RX series range of Clinical Chemistry Analysers offer the most comprehensive testing profile for assessing inflammation and infection complications which identifies any potential infectious issues a patient may have. The world famed RX series test menu includes specific tests associated with inflammation and infection including Alpha-I Acid Glycoprotein, ASO, CRP, Lactate and Rheumatoid Factor tests.
The RX series zinc test will assess the levels of zinc in a patient sample, zinc plays a significant role in an individual’s health. It’s functions include cell and enzyme production as well as wound healing, therefore it is vitally important for a patient to be aware if they are either deficient or within the normal range for zinc and other infectious related tests to ensure potential infections can be prevented. To view the full RX series test menu click here.
Internal Quality Control

Randox has partnered up with Qnostics to provide a wide range of molecular controls for infectious disease testing. Designed to meet the demand of today’s molecular diagnostics laboratory and laboratories carrying out Nucleic Acid Testing (NAT), the Qnostics Molecular Infectious Disease range comprises hundreds of characterised viral, bacterial and fungal targets covering a wide range of Transplant Associated Diseases, Respiratory Infections, Blood Borne Viruses, Sexually Transmitted Infections, Gastrointestinal Diseases and Central Nervous System Diseases.
External Quality Assessment

Randox have also partnered up with QCMD to offer a vast array of molecular EQA programmes for infectious disease testing. With an extensive database of over 15 000 participants in over 120 countries, QCMD is one of the largest providers of molecular EQA in the field of molecular diagnostics. Frequent challenges, comprehensive reports and international accreditation ensures the best assessment of test system performance.
Randox Biochip Array (BAT) Technology free from Biotin-Streptavidin
![Biotin - landing page banner[2] Biotin](https://www.randox.com/wp-content/uploads/2018/07/xBiotin-landing-page-banner2.jpg.pagespeed.ic.33PpDA8fvo.jpg)
Biotin, also known as vitamin B7, is involved with fatty acid metabolism, amino acid degradation, and gluconeogenesis. The recommended daily intake for biotin is roughly 30-70µg, which is extremely low, meaning that biotin deficiency is rare. Recently, there has been a surge in biotin supplementation mainly for beauty reasons, including: stronger nails and healthier skin and hair, resulting in the biotin craze on Instagram. Currently 162K posts are attributed to the biotin hashtag (#biotin) on Instagram. Whilst biotin supplementation is beneficial for numerous health conditions, including: multiple sclerosis (MS), diabetes, elevated cholesterol, and metabolic dysfunction, the increasing use of biotin by patients has created a problem with in vitro diagnostic testing.
With numerous manufacturers using biotin-streptavidin technology to develop in vitro diagnostic tests, combined with the rise in biotin supplementation use, the FDA (food and drug administration) issued an alert regarding the potential for erroneous results triggered by high levels of biotin in patient samples, at the end of 2017. Clinical decisions based on these false results from biotin technology can lead to inaccurate diagnosis and inappropriate treatment prescribed. The FDA confirmed that a patient, who was consuming high levels of biotin, died when a troponin tested was skewed and failed to show that the patient was having a heart attack. Other tests that can produce erroneous results include: cardiac, pregnancy, cancer and iron-deficiency tests.
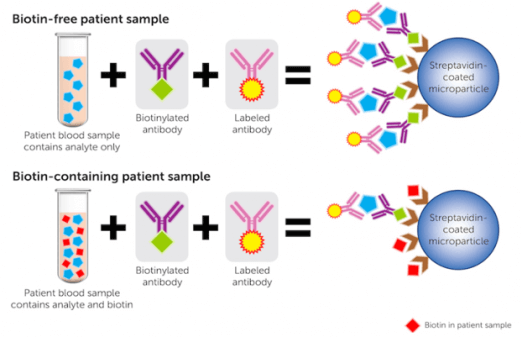
(Halasey, 2018)
The image above highlights that in biotin-free patient samples, the analyte to be tested successfully binds to the biotinylated antibody and the labelled antibody ultimately ensuring accurate measurement. In the patient sample containing high levels of biotin, the biotin inhibits streptavidin’s ability to capture the analyte-antibody complex, generating falsely lowered results.
As 70% of all clinical decisions are based on results from in vitro diagnostic tests, it is vital that laboratories are selecting in vitro diagnostic tests that do not adopt the biotin-streptavidin technology to ensure accurate patient testing.
Randox do not utilise the biotin-streptavidin technology in the development of the Biochip Array Technology (BAT).
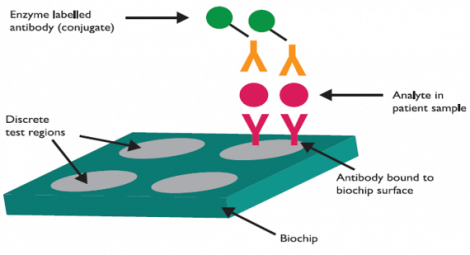
The Randox Biochip facilitates multiplex testing for faster, more comprehensive patient testing. This technology, free from biotin-streptavidin, uses capture antibodies bound to a solid substrate (biochip surface) as opposed to biotinylated antibodies used by other manufacturers. The Biochip also utilises enzyme labelled conjugate to enable chemiluminescent detection of the target in the patient sample.
Biochip test menu

The Biochip Array Technology (BAT) from Randox is capable of simultaneous multi-analyte diagnostic testing within the fields of clinical research and drugs of abuse testing. The technology works through combining a panel of related assays on a single biochip with a single set of reagents, controls and calibrators. An extensive range of Biochip panels are available, each optimised to provide the best performance.
Evidence
The evidence analyser is the world’s first protein Biochip Array Technology system and has truly transformed laboratory diagnostics worldwide. As the first of its kind, the Evidence has introduced higher standards of quality efficiency and reliability to numerous sectors including hospitals and clinical laboratories, forensic and clinical toxicology, pharmaceutical/CRO applications, as well as veterinary laboratories.
Evidence Evolution

The world’s first fully automated random access Biochip testing platform. The Evidence Evolution is set to revolutionise current diagnostic testing. With the capability to process up to 2,640 tests per hour, the Evidence Evolution utilises multiplexing technology, offering advanced test consolidation, patient profiling, a complete system integration, as well as the most comprehensive test menu on the market.
Investigator

The #1 choice for research, clinical, forensic, and veterinary testing. Using the multiplexing technology, the semi-automated benchtop immunoanalyser Evidence Investigator is suitable for medium throughput laboratories. In addition to the current test menu for this analyser, Randox have new tests in development.
Reference:
Halasey. S, 2018, “Inside Track: Biotin Gets a Safety Alert”. Available from URL: http://www.clpmag.com/2018/01/inside-track-biotin-gets-safety-alert/
Randox – Nutritional Status

Randox are dedicated to improving health worldwide, throughout the month of July we hope to highlight how the Randox clinical product range can assess an individual’s nutritional status both accurately and swiftly allowing the necessary steps to be taken in order to improve nutritional status. Nutritional status is influenced by diet, the levels of nutrients in the body and the body’s ability to maintain normal levels of metabolic integrity. Assessing nutritional status will generally indicate if an individual is eating the correct variety and quantity of nutrients. The Randox portfolio comprises a wide range of products to combat nutritional status issues including the RX series range of biochemistry analysers, diagnostic reagents such as zinc, copper & lipase and an extensive QC range catering for nutritional status testing in both liquid & lyophilised formats.
Nutritional Status: Reagents

Randox Reagents have the highest quality reagents on the market and a test menu comprising of over 113 assays. Unique to Randox is the copper and zinc assays that hold equal importance in the diagnosis of kidney and liver damage.
The Randox zinc assay can be used to measure levels of zinc in a patient’s urine providing insight into the levels of zinc in the body. The Randox copper assay is used to measure the levels of copper in the blood in order to determine copper toxicity. Combining these Randox assays can aid in identifying the cause of liver damage in a patient, leading to correct treatment and recovery.
Nutritional Status: RX series

The RX series range offers the most comprehensive testing profile for assessing nutritional status to identify any nutritional deficiencies or any other nutritional issues within an individual. The world famed RX series test menu possesses the most extensive nutritional status testing panel available to give an expansive picture of an individual’s nutritional status. The RX series zinc test can identify a zinc deficiency in an individual which is often a result of a low dietary intake and can lead to many problems including impaired immune & cognitive functions, kidney disease and diabetes. To view the full RX series test menu click here.
Nutritional Status: Quality Control

The Randox Acusera Liquid Chemistry Premium Plus control is designed for use in the routine monitoring of reproducibility and is the most comprehensive chemistry control available. As a true third party control it is suitable for use with all major instruments and covers over 100 parameters including routine chemistries, immunoassays, lipids, cardiac markers, proteins, therapeutic drugs and electrophoresis effective consolidation and significant cost savings are guaranteed.
Nutritional Status: RIQAS

The RIQAS General Clinical Chemistry EQA programme is designed to monitor the performance of up to 52 parameters covering routine chemistries, lipids, hormones and trace metals. Three flexible, cost effective programme options allow you to choose the correct challenge that is right for your lab. Bi-weekly, online reporting with reports returned within 72 hours allows corrective action to be taken sooner, avoiding dangerous misdiagnoses.
Improving Women’s Health With Randox

Randox are dedicated to improving health worldwide, Throughout the month of June we hope to highlight how the Randox clinical product range can ensure accurate and swift diagnosis and by doing so improving women’s health by allowing for the necessary steps to be taken post diagnoses. A common held misconception is that breast cancer is the biggest threat to a women’s health however the biggest health risk to a woman statistically is heart disease which accounts for roughly 27% of female death. The Randox clinical product range offers a wide range of products to combat heart issues including the RX series extensive cardiac testing panel, reagents such as H-FABP, Adiponectin an TxB Cardio and an extensive cardiac QC range available in both liquid & lyophilised format.
Women's Health: Reagents

Randox Reagents have the highest quality reagents on the market and a test menu comprising of over 118 assays covering over 100 disease markers. Several of these reagents will play a key role in diagnosis in woman such as Randox’s Lipoprotein (a) – Lp(a) test, The Randox Lp(a) offers swift and accurate diagnosis of elevated circulation Lp(a) levels which is significant for woman as women have an increased risk of CVD due to elevated levels of Lp(a). Several traditional CVD risk markers, including elevated LDL may be absent in some women, elevated Lp(a) levels may identify women at high risk of developing CVD.
Women's Health: RX series

The RX series range of clinical chemistry analysers offers the most comprehensive testing profile for assessing health in males and females. Urinary tract infections are more commonly found in women and the RX series extensive renal function panel will provide clarity in terms of a woman’s urological health by testing for 19 separate analytes, including microalbumin. The RX series microalbumin test can detect very low levels of albumin in urine and if albumin is detected it can be an indicator of kidney injury and can result in irreversible damage. To view the full RX series test menu click here.
Women's Health: Quality Control

The Randox Acusera Maternal Screening quality control is the only commercially available control which covers all six analytes used during first and second trimester screening of Down’s syndrome and Spina Bifida. Instrument specific target values and ranges are provided for AFP, Inhibin A, PAPP-A, β hCG, Total hCG and Unconjugated Estriol. The inclusion of PAPP-A and Inhibin A eliminates the need to purchase additional controls at extra expense.
Women's Health: RIQAS

The RIQAS Maternal Screening EQA programme is designed to monitor the performance of screening tests used during the first and second trimester of pregnancy to assess the risk of Down’s syndrome, Spina Bifida and Trisomy 18. 100% human serum ensures commutability while the lyophilised material allows for enhanced stability. Monthly reporting allows laboratories to become aware of issues and remedy them early.
Protected: About Randox
Neonatal Health With Randox

Neonatal screening is vital for the overall health of newborn babies. Although it is not a mandatory procedure it is highly recommended to detect infections and diseases that can have devastating consequences if not diagnosed soon after birth. Randox’s range of neonatal reagents include CRP, Bilirubin and Complement C4 that are essential in the detection of neonatal lupus and jaundice; which if left undetected can lead to sickle cell anaemia, rubella and even syphilis. Armed with these specific and high quality reagents and Randox Quality Control material ensures not only the early detection of such diseases but also the accuracy and reliability of the laboratory test results that can help to ensure that your baby gets the best start in life.
Neonatal Health: Reagents

Randox Reagents have the highest quality reagents on the market and a test menu comprising of over 118 assays covering over 100 disease markers. Our specific reagents for neonatal health include, G-6-PDH, IgE, Bilirubin, Copper, Complement C4 & C3, IgA , CRP and CRP full range. With flexible pack sizes and a comprehensive list of analyser applications available, you are sure to find what you are looking for.
Neonatal Health: RX series

Randox has developed the RX series of clinical chemistry analysers for superior semi-automated and fully automated testing. The RX series extensive dedicated test menu goes beyond routine testing and has many unique and high-performance tests available. Our range of tests covers several parameters to assess neonatal health.
Neonatal Health: Quality Control

Randox Liquid Bilirubin Control provides a true third party solution for the measurement of bilirubin. Designed to deliver unbiased and independent results, it ensures your instrument is accurate and reliable when testing real patient samples. An elevated bilirubin control is also available to monitor accuracy at higher levels enabling early medical intervention when it matters.
Neonatal Health: RIQAS

RIQAS is the largest international external quality assessment scheme with 45,000 participants worldwide. With 33 comprehensive EQA programmes and world renowned consolidation, it reduces the number of individual programmes required. RIQAS programmes are available to compliment neonatal health tests.
NeoNatal

Metabolic health is a term used to describe a collection of required chemical reactions that take place in all living organisms. A metabolic disorder develops when an abnormal chemical reaction occurs which alters the normal metabolic process.
Randox has developed the RX series of clinical chemistry analysers for superior semi-automated and fully automated testing. The RX series extensive dedicated test menu goes beyond routine testing and has many unique and high-performance tests available. Our range of tests covers several parameters to assess your overall metabolic health.
Metabolic Health Profile
| Albumin | Chloride | Potassium |
| Alkaline Phosphatase | C02 Total | Sodium |
| ALT | Creatinine | Total Bilirubin |
| AST (GOT) | Glucose | Total Protein |
| Direct Bilirubin | Lactate | Urea |
| Calcium |
The RX series clinical chemistry analysers provide laboratories with a robust and smart solution ensuring you maintain a consistent workflow and can provide accurate results first time, every time. Offering excellent customer support services, our trained engineers are on hand to work with you in preserving the continuity of your operations while maximising the potential of your RX series instrument.Our world-famous test menu of high quality reagents ensures excellence in patient care, guaranteeing unrivalled precision and accuracy reducing costly test re-runs or misdiagnosis and offering complete confidence in results.
For more information visit: https://www.randox.com/clinical-chemistry-analysers/
Metabolic syndrome comprises of risk factors such as high blood pressure, high blood sugar/cholesterol and abdominal fat, which together, can lead to serious long term problems including an increased risk of heart disease, stroke and developing type 2 diabetes. Randox have developed an extensive range of reagents to accurately diagnose and monitor metabolic health, with the aim that patients can take the appropriate measurements to improve their health.
All reagents are available for use on a wide range of third party biochemistry analysers. Contact us to request an application for your specific analyser.
Adiponectin
Adiponectin is a clinical diagnostic biomarker for metabolic risk assessment. Adiponectin is solely excreted by adipocytes and is a protein hormone with anti-inflammatory and insulin-sensitising properties. It plays an important role in a number of metabolic processes such as glucose regulation and fatty acid oxidation. Levels of adiponectin have been linked with several pathologies including metabolic syndrome, cancer and cardiovascular disease, as levels are inversely correlated with Abdominal Visceral Fat (AVF). Randox is one of the only manufacturers of adiponectin in an automated biochemistry format, removing the inconvenience and time consumption associated with traditional ELISA-based testing.
For more information visit: https://www.randox.com/adiponectin/
Small-dense LDL (sLDL) Cholesterol
Small-dense LDL cholesterol is a vital cardiac risk marker, usually tested in patients with prediabetes or metabolic syndrome. Randox sLDL utilises the “Denka Seiken” method which produces results in less than ten minutes. Research has shown individuals with a predominance of sLDL have a 3-fold increased risk of myocardial infarction (MI), even for those who HDL, LDL, Total Cholesterol and Triglyceride levels are considered ‘normal’. Elevated levels of sLDL are associated with a sedentary lifestyle, a diet high in saturated fat, insulin resistance, pre-diabetes and genetic disposition. Measurement of sLDL allows the clinician to get a more comprehensive picture of lipid risk factors and tailor treatment accordingly. Reducing LDL levels reduce the risk of both CVD and MI.
For more information visit: www.randox.com/sldl-cholesterol/
TxBCardio™
Randox TxBCardio™ is used to assess the level of aspirin resistance in patients at risk of cardiovascular disease. Aspirin resistance is a serious clinical problem and is estimated to affect 25-20% of patients on a low dosage. It is important to assess aspirin resistance as this may be anticipated in patients with metabolic syndrome. The identification of these patients can be significantly improved through the use of Randox TxBCardio™ as the results generated can be used to enable timely intervention by clinicians and they can focus on anti-platelet therapy.
For more information visit: https://www.randox.com/txbcardio/
Other metabolic health tests:
- Albumin
- Alkaline Phosphatase
- ALT
- AST (GOT)
- Direct Bilirubin
- Calcium
- Chloride
- CO2 Total
- Creatinine
- Glucose
- Lactate
- Potassium
- Sodium
- Total Bilirubin
- Total Protein
- Urea
When diagnosing and monitoring metabolic-related complications such as Diabetes and Cardiovascular Disease (CVD), it is vital that laboratories have a robust Quality Control (QC) system in place to ensure the accuracy and reliability of the results produced. As a world leading manufacturer of QC solutions including Third Party Controls and External Quality Assessment (EQA), our extensive product portfolio is designed to help reduce costs and time without compromising on quality.
Diabetes
Liquid HbA1c Control
- Liquid ready-to-use format ideal for use in the lab or at the POC
- Whole blood sample matrix commutable with that of the patient sample
- Assayed control with values available for HPLC
- Convenient bi-level pack accurately covering the patient reportable range
- Open vial stability of 30 days ultimately helping to reduce waste and costs
- For more information visit: https://www.randox.com/liquid-hba1c-quality-control/
Glycated Hemoglobin EQA Programme
- Monthly analysis ensuring early identification of test system errors
- Maximised peer groups for comparative performance assessment
- Reports available within 72 hours allowing corrective action to be taken immediately
- User-friendly reports delivering at-a-glance performance assessment
- For more information visit: https://www.randox.com/hba1c-eqa/
CVD risk
Lipid Control
- Covers the complete lipid profile
- True third party control ensuring unbiased performance assessment with any instrument or method
- Manufactured from 100% human serum ensuring a matrix commutable with the patient sample
- Free from Sodium Azide which can interfere with direct clearance methods of HDL and LDL Cholesterol
- Three clinically significant levels available covering low, borderline and high risk levels of HDL and LDL Cholesterol
- For more information visit: https://www.randox.com/lipid-quality-control/
- Covers the complete lipid profile with a choice of reporting just three parameters at a reduced cost
- Monthly analysis ensuring early identification of test system errors
- Maximised peer groups for comparative performance assessment
- Reports available within 72 hours allowing corrective action to be taken immediately
- User-friendly reports delivering at-a-glance performance assessment
- For more information visit: https://www.randox.com/lipids-eqa/
Our Randox Health clinics offer comprehensive health testing as part of our Signature, Everyman and Everywoman packages to give you a comprehensive picture of your overall metabolic health. Our clinics utilise the same cutting-edge tests and quality control that we have available to clinical laboratories globally, as well as our patented Biochip Array Technology (BAT).
For more information visit: https://www.randoxhealth.com/
A common misconception surrounding metabolic health is that it refers solely to your weight, and if you are overweight you are considered to be unhealthy. But in actual fact this may not be entirely true. Good metabolism means that your body is in good overall health, which doesn’t account for just your weight! Common metabolic disorders include genetic metabolic disorders, diabetes and metabolic syndrome. Understanding and testing to see how well your metabolism is functioning is key to ensuring long lasting health.
Genetics
There are a number of genetic metabolic disorders caused by mutations of single genes. Examples of common disorders include Gaucher’s disease, hemochromatosis and cystic fibrosis. Gaucher’s disease is a genetic disorder that affects the body’s ability to break down fat that can accumulate in the liver/spleen and bone marrow. Hemochromatosis is a condition that is caused by the over-absorption and build-up of iron while cystic fibrosis is a metabolic disorder that appears as a result of a build-up of mucus in lungs/liver and intestines. Each of these metabolic disorders affect certain organs from functioning properly and therefore your overall healthiness.
Diabetes
Type 2 diabetes is one of the most common types of metabolic disorders in the world that is expected to affect 592 million people by 2035. It is characterised by high blood sugar, insulin resistance or a lack of insulin being produced by the pancreas. Insulin resistance occurs when the body isn’t able to use insulin the right way which increases blood glucose levels. Insulin is needed for cells to take in glucose (sugar) from the bloodstream and convert it into energy. Over time this lack of insulin can damage the organs in your body.
Metabolic Syndrome
Metabolic syndrome (also known as syndrome X, Reaven’s syndrome, and CHAOS) is not a disease but a collection of risk factors that affect your health; these include high blood pressure, high blood sugar/cholesterol and abdominal fat. Left untreated, these risk factors, together, can lead to long term serious problems including an increased risk of heart disease, stroke and developing type 2 diabetes.
Can you improve your metabolic health?
Yes! The good news is that if you discover that your metabolic health is not up to scratch you can improve it through a combination of diet, exercise and lifestyle adjustments such as:
- 30 minutes of moderate to intense exercise 5-7 times a week
- Low-dose aspirin to reduce your risk of stroke or heart attack
- Quit smoking
- Medication for blood pressure/cholesterol/ blood sugar
- Limit alcohol intake
- Eat a healthy balanced diet
