Randox Testing Services – Drug and Alcohol Testing
Randox Testing Services – Drug and Alcohol Testing
At Randox Testing Services, we provide reliable and cost-effective drug and alcohol testing solutions for a wide range of industries. Sectors we work with include aviation, maritime, manufacturing, and more. Additionally, our Collection Officer Network operates 24 hours a day, 365 days of the year with a target call-out time of 2 hours. With over 40 years of experience in the diagnostics industry, our services not only ensure safety but also compliance in workplaces across the UK and Ireland.
We understand that every industry has unique needs, which is why we craft customised testing packages to meet specific workplace requirements. Whether it’s random testing to deter substance abuse or post-incident testing following an accident, we offer flexible solutions that cater to your business’s safety priorities. Furthermore, our expertise is trusted by industries ranging from safety-critical sectors to the medico-legal field such as family law, ensuring that our clients receive accurate, reliable, and legally defensible results.
- Pre-Employment Testing: Essential for ensuring candidate suitability and maintaining a safe environment for your current workforce.
- Random Testing: A highly effective deterrent for substance abuse, as employees are aware they could be tested at any time, ensuring integrity across all levels of the organisation.
- With-Cause Testing: Conducted when there is suspicion or allegations of drug or alcohol abuse in the workplace, or when drugs and alcohol have been found.
- Post-Incident Testing: Occurs after an accident or incident to determine if drugs or alcohol played a role.
- Abstinence Testing: Used to monitor employees who previously tested positive to ensure they remain abstinent from substance misuse.
We utilise discreet and non-invasive methods for sample collection, offering options such as urine, hair, oral fluid, breath or a combination of these. This enables both short-term and long-term drug profiling. Moreover, our testing methods are designed to suit your organisation’s needs and ensure accuracy and reliability in detecting drug and alcohol use. Each sample is handled following strict chain of custody procedures to guarantee sample integrity from collection to reporting.
We follow a thorough and professional process to deliver tailored drug and alcohol testing solutions:
-
- Consultation: We start with a free consultation to discuss your requirements and create a customised testing package.
- Policy Creation: We help develop a clear and concise workplace drug and alcohol policy that outlines employer rights while protecting employees.
- Policy Implementation: Our training and educational services help you effectively communicate the policy to your team.
- Sample Collection: Our trained officers collect samples at a time and location that suits you, maintaining sample integrity throughout the process.
- Sample Analysis: Using our advanced Biochip Array Technology, we screen samples for a wide range of substances, ensuring reliable and accurate results.
- Results Reporting: Results are delivered confidentially via our secure web portal, with options for alternative methods if preferred.
We test for a wide range of drugs across different panels using both instant testing kits and lab-based confirmatory analysis. This flexibility allows us to meet the diverse needs of our clients, ensuring all substances of concern are covered. Substances include:
| Substances Tested | |||
|---|---|---|---|
| Amphetamine | Phencyclidine | Alcohol | LSD |
| Buprenorphine | Cannabinoids | Barbiturates | Opioids |
| Benzodiazepine | Oxycodone | Benzodiazepine 1 | Mephedrone |
| Cocaine Metabolite | Tramadol | Benzodiazepine 2 | Pregabalin |
| Methadone | EDDP | Methaqualone | Gabapentin |
| Methamphetamine | Ketamine | Fentanyl | Zolpidem |
| Opiates | MDMA/Ecstasy | Propoxyphene | Zopiclone |
- Expertise across various industries, including workplace and medico-legal
- Accurate and reliable testing methods
- Global network of trained sample collection officers
- Customised training and education seminars
- Free policy review and consultation
Randox Testing Services is committed to helping businesses enhance workplace safety, reduce absenteeism, and ensure compliance with drug and alcohol regulations. Contact us today at testingservices@randox.com or call +44 (0) 28 9445 1011 to speak with one of our experts.
Understanding Xanthochromia
Understanding Xanthochromia
When faced with a potential subarachnoid haemorrhage (SAH), the tools we use to diagnose can quite literally be life-saving. Cerebrospinal fluid (CSF) analysis plays a pivotal role, especially when common diagnostic tools like Computed Tomography (CT) scans might not catch early signs.
Traditionally, xanthochromia detection relied on visual assessment – a method that suffers from inconsistency due to subjective interpretation and lacks uniformity across the industry. Today, spectrophotometry has emerged as the preferred method for its precision and reliability in detecting xanthochromia. To ensure the highest accuracy, this technique requires stringent quality control measures. Here, we discuss xanthochromia and SAH, before introducing our dedicated Xanthochromia true third-party control.
What is Xanthochromia?
Xanthochromia, derived from the Greek word, ‘xanthos,’ meaning yellow, refers to the yellow, or sometimes pink, discolouration of CSF, primarily due to bilirubin, a by-product of haemoglobin breakdown. Why does this matter? Because it’s a tell-tale sign of bleeding within the brain, often indicating SAH when CT scans don’t. Understanding this can help us catch and treat critical conditions before they worsen. Xanthochromia may also be an indicator of intracerebral haemorrhage, brain tumours, infection, or severe systemic jaundice1.
Subarachnoid Haemorrhage
SAH is a spontaneous intracranial bleed characterised by significant mortality and morbidity rates. Approximately 12% of patients die before receiving medical attention, 33% within 48 hours, and 50% within 30 days of an SAH. Among the survivors, half suffer from permanent disabilities, with an estimated lifetime cost more than double that of an ischemic stroke2. Patients which have displayed symptoms often complain of severe headache, nausea, vomiting, photophobia and/or phonophobia3.
CT scans, particularly non-contrasted CTs of the brain or CT angiograms (CTAs), are often the first line of diagnostic tools for suspected SAH. However, up to 5% of SAH cases may not show any signs of haemorrhage on these scans within the first 24 hours, with this figure rising to 50% by the end of the first week and remaining around 30% by the second week4.
In contrast, xanthochromia in the CSF can be detected as early as two hours after a bleed and is observed in over 90% of patients within 12 hours of an SAH event. This detection can persist for up to three to four weeks, offering a critical diagnostic window that imaging alone might miss. The conversion from haem to bilirubin in CSF takes roughly 6 to 12 hours, suggesting that xanthochromia is most reliably identified between 6- and 12-hours post-bleed. More than 75% of patients may still present with xanthochromia at 21 days following an SAH1.
Pathophysiology explained
A ruptured cerebral aneurysm will begin to leak blood into the CSF. This blood is gradually degraded by macrophages to yield various by-products including oxyhaemoglobin, which is subsequently converted to bilirubin in a process lasting between 6 and 12 hours1. Crucially, this conversion to bilirubin can only occur in vivo, providing a unique marker for diagnosing subarachnoid haemorrhage when observed in the CSF1.
The Importance of Accurate Detection
In many parts of the world, including the US, visual detection remains a common initial test for xanthochromia in CSF.
- Procedure: Spinning a CSF sample in a centrifuge and comparing the supernatant against a vial of water, held against a white backdrop to detect a yellow or pink tint.
- Indication: A change in colour indicates that blood has been present in the spinal fluid for at least two hours, with all patients showing signs by 12 hours post-bleed1.
However, this method is prone to false positives due to:
- Dietary influences: High intake of carotenoids (like carrots and spinach).
- Medication: Use of Rifampin.
- Medical conditions: Clinical jaundice or high protein levels in CSF, which can be seen in conditions like carcinomatosis and meningitis1.
Spectrophotometry
Spectrophotometry offers a more precise alternative by measuring light absorption in materials at specific wavelengths:
- It can detect the presence of bilirubin, which absorbs light at 440 to 460 nm, a definitive indicator of xanthochromia.
- Advantages over visual detection: This method eliminates the interference from other pigments or proteins and can distinguish bilirubin from oxyhaemoglobin, crucial for accurate diagnosis.
Quality control is crucial in spectrophotometry to ensure the accuracy and reliability of xanthochromia tests:
- Regular Maintenance: Routine checks and maintenance of the spectrophotometer are fundamental to its operation. This helps in maintaining the instrument’s precision in measuring light absorption at specific wavelengths crucial for detecting bilirubin in CSF.
- Calibration: Calibrating the spectrophotometer with known standards is essential. This process adjusts the instrument to measure the absorption accurately, particularly vital given bilirubin’s narrow detection window between 440 and 460 nm.
Implementing these stringent QC measures enhances the diagnostic precision of spectrophotometry, boosting confidence in the results. Such practices ensure that patients are diagnosed accurately and receive timely, appropriate treatment, solidifying the value of advanced diagnostic techniques in medical settings.
Introducing Randox Xanthochromia Controls
Diagnosing SAH swiftly and precisely is critical due to its significant immediate and long-term impacts. To aid precise detection, our Liquid Frozen Xanthochromia Positive & Negative Controls are essential tools for laboratories conducting CSF analysis. Here’s what makes them stand out:
- Dedicated Xanthochromia true third-party control with only 2 analytes for limited cross-reactivity – Bilirubin & Oxyhaemoglobin
- 2-day open vial stability at 2° to 8°C and a 11-week shelf life from date of manufacture when stored at -18ºC to -24ºC.
- Liquid frozen control provides suitable matrix in an easy-to-use format.
- Consistent, clinically significant values.
- Suitable for use with UV spectrophotometers, these controls help monitor bilirubin and oxyhaemoglobin levels effectively.
The Randox Xanthochromia Controls are ideally suited for laboratories, both public and private, as well as researchers who perform CSF analysis. Their use is crucial in ensuring the precision of SAH testing, which contributes to more accurate diagnostics and ultimately leads to better patient outcomes.
Considering the crucial role of accurate xanthochromia detection in diagnosing SAH, isn’t it time to review your lab’s capabilities? Explore how Randox Xanthochromia Controls can enhance your diagnostic processes. For more details on how to get these tools in your lab, contact us at marketing@randox.com.
In the fight against conditions like SAH, every second and every test counts. Equip your lab with Randox Xanthochromia Controls to ensure that your diagnostics are as precise and reliable as possible, helping save lives and improve healthcare outcomes.
References
-
- Dugas C, Jamal Z, Bollu PC. Xanthochromia. StatPearls Publishing; 2024. Accessed August 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526048/
- Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97(1):14-37. doi:10.1016/j.pneurobio.2012.02.003
- NHS. Subarachnoid haemorrhage. https://www.nhs.uk/conditions/subarachnoid-haemorrhage/.
- Chakraborty T, Daneshmand A, Lanzino G, Hocker S. CT-Negative Subarachnoid Hemorrhage in the First Six Hours. Journal of Stroke and Cerebrovascular Diseases. 2020;29(12):105300. doi:10.1016/j.jstrokecerebrovasdis.2020.105300
Active vs Total Vitamin B12
Total vs Active B12
Vitamin B12, or cobalamin, is a vital water-soluble vitamin that plays an essential role in myelination initiation and development, cellular energy and fatty acid metabolism. It is a cofactor for enzymes methionine synthase and L-methyl-malonyl-coenzyme A mutase and, in addition to folate, is essential for DNA and protein synthesis. In the UK, up to 6% of adults under 60 have been diagnosed with Vitamin B12 deficiency and figures are much higher in elderly populations1. Additionally, these data do not consider the high rates of missed diagnosis associated with B12 deficiency, which some reports claim to be as high as 26%2. New guidance from the National Institute of Health and Care Excellence (NICE) advise that Active vitamin B12 testing is recommended for some groups of patients. In this article, we’ll look at this essential vitamin, B12 deficiency and the associated complications, compare the biomarkers used to diagnose B12 deficiency, and finally, present the new Acusera Active B12 Control.
Aetiology
Vitamin B12 deficiency can arise due to dietary insufficiency, malabsorption resulting from damage to the small intestine, often caused by conditions like Coeliac disease or Crohn’s disease, or via pernicious anaemia – an autoimmune condition which results in an inability to absorb vitamin B12.
It is a common problem in the elderly population – bodily stores of vitamin B12 can take up to 20 years to become depleted, meaning complications have often already begun before diagnosis occurs. The most common source of vitamin B12 comes from dietary intake of animal products therefore vegetarian dietary requirements are considered a considerable risk factor for vitamin B12 deficiency.
Pathophysiology and Complications
Vitamin B12 deficiency significantly impacts health, affecting various bodily functions, potentially leading to a range of complications. Megaloblastic anaemia is a common complication associated with vitamin B12 deficiency and is characterised by the presence of large red blood cell precursors (megaloblasts) in the bone marrow3. The lack of vitamin B12 results in impaired DNA synthesis and an inhibition of nuclear division. However, cytoplasmic maturation is less effected. This results in asynchronous maturation of the nucleus and cytoplasm in erythrocytes and causes the synthesis of abnormally large megaloblasts. This causes the cessation of DNA synthesis and DNA replication errors, culminating in apoptotic cell death. Common symptoms of megaloblastic anaemia include weakness, shortness of breath, palpitations, tachycardia, Hunter glossitis or splenomegaly3.
Pernicious anaemia is a condition commonly associated by vitamin B12 deficiency. Pernicious anaemia is an autoimmune disorder which affects the gastric mucosa resulting in impaired absorption of vitamin B12. Common symptoms of pernicious anaemia include glossitis, hair loss, dry skin, memory loss, poor concentration, poor sleep, confusion and dizziness, shortness of breath, Diarrhoea, indigestion, loss of appetite, mood swings and suicidal thoughts.
Neurological issues may also arise, including numbness, mobility loss, and memory issues, and in some cases, depression4. Additionally, B12 deficiency is linked to increased risks of cardiovascular events5, infertility6, and autoimmune diseases like multiple sclerosis7 and lupus8. In children, vitamin B12 deficiency can manifest as failure of brain and overall growth and development, developmental regression, hypotonia, lethargy, hyperirritability, or coma9.

Active B12 as a marker of Deficiency
There are several markers of vitamin B12 deficiency. The most used in clinical practice are total vitamin B12, homocysteine, methylmalonic acid (MMA), and Holotranscobalamin (HoloTC) – also known as Active B12. HoloTC accounts for between 10-30% of total B12 and is the metabolically active form of vitamin B12.
When compared with total B12 quantification, HoloTC measurement has been shown to be a more sensitive and specific biomarker of B12 deficiency, particularly at borderline clinical levels10, in various cohorts11,12 including those on vegan diets13 – a known risk factor for B12 deficiency. Furthermore, HoloTC was shown to provide the higher diagnostic accuracy in clinical and subclinical B12 deficiency versus Total B12, MMA and homocysteine with significantly higher accuracy in women over 5011 – a population at high risk of B12 deficiency.
In response to the mounting evidence of the superior utility of HoloTC quantification, the National Institute for Health and Care Excellence (NICE) have produced new guidelines recommending either total B12 or HoloTC for the initial testing of suspected vitamin B12 deficiency. These guidelines specify the use of active B12 during pregnancy and suggest that active B12 might provide a more specific assessment in certain clinical contexts.
Acusera Active B12 Control
For the reasons stated above, Randox are proud to present the Acusera Active Vitamin B12 Control. This control is designed for use with in vitro diagnostic assays for the quantitative determination of HoloTC in human serum and plasma and is suitable for use on a variety of analysers. This true third-party control is provided in a liquid ready-to-use format reducing preparation time and has an impressive 30-day open vial stability, helping to minimise waste. Like all Acusera controls, the Active B12 Control is supplied at consistent, clinically relevant levels to ensure the test system is challenged at the critical decision limits used to aid diagnosis. Furthermore, this control is provided with assayed target values for a range of analysers which are available through our new SmartDocs portal.
Summary of Benefits:
- Dedicated, HoloTC control.
- 30-day Open Stability.
- 2-year shelf life.
- Liquid Ready-to-use.
- Human Serum Based.
- Consistent, clinically significant values.
- True third-party controls.
- Assayed target values.
Ensure the accuracy of your vitamin B12 testing with Randox’s Acusera Active Vitamin B12 Control. Join the other laboratories around the world who trust Acusera to help deliver reliable, clinically relevant test results. Contact us today at marketing@randox.com to learn more and order your supply of the Acusera Active B12 Control.
References
- Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349(sep04 1):g5226-g5226. doi:10.1136/bmj.g5226
- Oh RC, Brown DL. Vitamin B 12 Deficiency Clinical Manifestations of Vitamin B 12 Deficiency. Vol 67.; 2003. www.aafp.org/afp
- Hariz A, Bhattacharya PT. Megaloblastic Anemia. StatPerals Publishing; 2024.
- Patel S V., Makwana AB, Gandhi AU, Tarani G, Patel J, Bhavsar V. Factors associated with vitamin B12 deficiency in adults attending tertiary care Hospital in Vadodara: a case control study. Egypt J Intern Med. 2022;34(1):11. doi:10.1186/s43162-022-00104-0
- Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr Rev. 2013;71(2):110-117. doi:10.1111/nure.12001
- Green R, Graff JP. Megaloblastic Anemia. In: Atlas of Diagnostic Hematology. Elsevier; 2021:47-51. doi:10.1016/B978-0-323-56738-1.00004-X
- Najafi MR, Shaygannajad V, Mirpourian M, Gholamrezaei A. Vitamin B(12) Deficiency and Multiple Sclerosis; Is there Any Association? Int J Prev Med. 2012;3(4):286-289.
- Segal R, Baumoehl Y, Elkayam O, et al. Anemia, serum vitamin B12, and folic acid in patients with rheumatoid arthritis, psoriatic arthritis, and systemic lupus erythematosus. Rheumatol Int. 2004;24(1):14-19. doi:10.1007/s00296-003-0323-2
- Stabler SP. Vitamin B12 Deficiency. New England Journal of Medicine. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
- Bondu JD, Nellickal AJ, Jeyaseelan L, Geethanjali FS. Assessing Diagnostic Accuracy of Serum Holotranscobalamin (Active-B12) in Comparison with Other Markers of Vitamin B12 Deficiency. Indian Journal of Clinical Biochemistry. 2020;35(3):367-372. doi:10.1007/s12291-019-00835-y
- Jarquin Campos A, Risch L, Nydegger U, et al. Diagnostic Accuracy of Holotranscobalamin, Vitamin B12, Methylmalonic Acid, and Homocysteine in Detecting B12 Deficiency in a Large, Mixed Patient Population. Dis Markers. 2020;2020:1-11. doi:10.1155/2020/7468506
- Verma A, Aggarwal S, Garg S, Kaushik S, Chowdhury D. Comparison of Serum Holotranscobalamin with Serum Vitamin B12 in Population Prone to Megaloblastic Anemia and their Correlation with Nerve Conduction Study. Indian Journal of Clinical Biochemistry. 2023;38(1):42-50. doi:10.1007/s12291-022-01027-x
- Lederer AK, Hannibal L, Hettich M, et al. Vitamin B12 Status Upon Short-Term Intervention with a Vegan Diet—A Randomized Controlled Trial in Healthy Participants. Nutrients. 2019;11(11):2815. doi:10.3390/nu11112815
Medical Laboratory Professionals Week 2024
Medical Laboratory Professionals Week (MLPW) is recognised every year in the last full week of April. It’s an opportunity to increase the public understanding of, and appreciation for, the hard work of clinical laboratory staff around the world. It’s also an opportunity to inject a little fun into the laboratory. So, this year, we’ve created a Lab Professionals QC Bingo card. Have a go and see how many your laboratory can get!
How many boxes does your lab tick?

If you’re calling Bingo! you must be an Acusera 24.7 customer. If not, keep reading to find out how you can make daily life in your laboratory more straightforward.
What are Medical Laboratory Professionals?
Medicine wouldn’t be where it is today without the work of these laboratory professionals. They’re on the frontline. Around 70% of medical decisions are based on results provided by medical laboratory staff. That’s a lot of pressure on the labs to make sure their results are accurate. Clinical laboratory staff not only perform the tests used to guide diagnosis and disease prevention, but they also check all the tests they use through rigorous quality control (QC) procedures.
This involves testing samples of known values to prove that the test system and its components perform as they should and provide accurate results. To do this, laboratories require QC material. It’s important that what’s in a QC is as similar to what you’d find in a patient sample as possible. This is known as commutability. Good commutability helps limit cross-reactivity in the test and inaccurate results.
It’s also important to make sure the QC material has concentrations of analytes at similar values to those used to make diagnostic decisions. If you wanted to validate the length of the ruler on your desk, it wouldn’t be helpful to set it down on a 100m running track. Similarly, when laboratory professionals want to ensure a test is producing accurate results, they want to test the system at the critical values used to make medical decisions so that they can be confident the results at these values are accurate.
Once lab staff have confirmed the accuracy of their tests, they can begin testing patient samples. For most people, what happens to a sample after it’s taken is a bit of a mystery. MLPW is the perfect opportunity to unravel this a little:
After your sample is collected, it gets sent over to the lab. Even just moving it there needs careful handling to make sure it’s still good for testing when it arrives. Once it’s in the lab, the team checks the equipment to make sure it’s working right and giving accurate results. The QC procedure varies depending on what they’re testing for, but they always make sure their tests are legitimate. Once they’ve checked everything and carried out the tests, a pathologist looks at the results to figure out what’s going on. They use this information to help decide on the best treatment plan for you.
Even this watered-down explanation makes it sound like a lot of work, right? At Randox, we recognise the vital role and dedicated efforts of medical laboratory professionals, and the invaluable contributions they make to society, and we hope that now, you do too.
Acusera 24.7
Bingo! That’s exactly how our customers feel when they realise how much time Acusera 24.7 can save them. Our innovative and intuitive QC data software is cloud-based, allowing you to log in from anywhere in the world to review your QC data.
Along with a wide range of interactive charts, including Levey-Jennings charts, Acusera 24.7 determines measurement uncertainty and sigma metrics for you, saving you the time and stress of manually calculating these tricky statistical analyses. And that’s just the beginning. Acusera 24.7 can link to LIMS for automated data entry, meaning lab staff don’t have to manual type long datasets, unless they want to of course; we also provide both semi-automated data upload and manual data entry options.
Access to a range of reports has never been easier. Acusera 24.7 is particularly useful when gaining or renewing your accreditation, and live peer group QC data, to give additional confidence in the accuracy of your results.
But this article is supposed to be about laboratory professionals, so we won’t bang on about it anymore. We just want everyone to know about Acusera 24.7 so they can get that daily bingo! feeling for themselves. If you want to learn more about our reports, charts, advanced statistical analysis, Acusera 24.7 more generally, or how Acusera 24.7 can help you achieve your accreditation, you can follow the links to the relevant blog post.
Last year, we interviewed two of our laboratory staff, Dean and Meadhbh, to find out what a normal day looked like for them. To find out what a day in the life of a laboratory professional is like, take a look at the interviews here
If you’d like to get in touch with us to discuss the advantages of Acusera 24.7, or you’ve made up your mind and want to get in on the action, reach out to us at marketing@randox.com. We’re always happy to brag about how great Acusera 24.7 is, and how we make life simpler for more and more laboratories every day.
RIQAS Performance Assessment – Z Score vs SDI
Z Score vs SDI
You work hard to implement top class quality control in all areas of your laboratory. The success of your labours is reported to you through your External Quality Assessment (EQA) results. It can be frustrating when your report is returned, only for you to find that you’ve been assigned a poor performance score due to other laboratories in your participation group.
At RIQAS, we want your EQA results to reflect your performance, not that of everyone else, to truly illustrate the efficacy of your quality control procedures. This is why, instead of Z scores, we report your performance in terms of a Standard Deviation Index (SDI). However, we know that in some countries, you’re required to report a Z score. Don’t fret. You can still find this result in the .csv file provided with your report.
A Z score is a statistical measurement that describes a value’s relationship to the mean of a group of values. In other words, it’s a value calculated to tell us how many standard deviations (SDs) a result is from the expected mean. Z score is reported in terms of SD’s, therefore a Z score of 0 shows the result is identical to the mean.
While useful in many cases, when used in EQA, a Z score can give a false perception of performance. We want RIQAS participant performance assessment to be based on their individual performance, rather than being impacted by how well, or poorly, the other laboratories in the group performed for a sample.
Z score is calculated using a variable SD. This means that as results are added, the mean and SD can change. For example, if overall performance for a sample improves, the CV associated with the data will decrease, causing an increase in Z score. Let’s take a quick look at how RIQAS performance assessment works, and then we can get into SDI.
RIQAS Performance Assessment.
Our target scoring system has been developed to provide a simple interpretation of your laboratory’s performance. To calculate a target score, your result is calculated as a percentage deviation (V) from the Mean for Comparison. This deviation is then compared to a Target Deviation for Performance Assessment (TDPA) to calculate the Target Score.
The difference between your result and the mean for comparison is expressed as a Target Score (TS) using the following mathematical formulae:

The better your percentage deviation compared to the TDPA, the higher your Target Score will be.

TDPA are set to encourage participants to achieve and maintain acceptable performance. Target Deviations are assigned to be fit-for-purpose and take all possible sources of variation into account, including sample homogeneity and stability as per ISO/IEC17043, ISO13528 and IUPAC.
In general, the TDPA is set so that ~10% laboratories achieve Target Scores less than 50. However, depending on homogeneity and stability, the TDPAs may be adjusted, so that participants’ performance is not adversely affected by sample variability. If your % deviation (V) is equal to the Target Deviation for Performance Assessment (TDPA) then a target score of 50 is achieved.
RIQAS reviews TDPAs annually and the methods used to assign them have been agreed by the RIQAS Advisory Panel.

Standard Deviation Index (SDI)
To provide a more accurate assessment of performance, we use SDI instead of Z score. SDI is a score which compares the participant’s difference from the assigned value (mean for comparison) with an evaluation interval called the Standard Deviation for Performance Assessment (SDPA).
The SDPA calculation involves a series of steps. First, we calculate a CV for Performance assessment (CVPA) as shown below:

As mentioned, the TPDA is normally set so that ~10% of laboratories achieve a TS less than 50. In such cases, the t-value used to convert TDPA to CVPA is ~1.645. However, depending on homogeneity and stability, the TDPA may need be increased, so that participants’ performance is not adversely affected by sample variability. In such cases less than 10% of laboratories will have poor performance, and a larger t-value will be chosen to convert TDPA to CVPA
We then convert CVPA to SDPA:

Using this equation, an initial SDPA is calculated for every mean for comparison (i.e. for all methods, method, and instrument statistics). However, for new parameters or those which have small participation numbers, it’s not always possible to assign a target deviation, TDPA or SDPA. In such cases, the SDPA will be the SD calculated when the mean for comparisons is generated.
According to ISO/IEC17043, when the assigned value is based on consensus (mean for comparison), the uncertainty of the assigned value must be calculated and combined with the SDPA when it is considered to be significant. This forms an adjusted SDPA, which is used to calculate the participant’s performance in terms of SDI.
Using the SDPAadjusted we can calculate SDI using the formula below:

On your RIQAS report, you’ll find the SDI associated with the current sample in the text section of each report page. We also provide your last 20 SDIs, plotted on a Levey-Jennings chart, along with an indication of the mean for comparison for each sample (I = Instrument group, M = Method group, or A = All Methods group). Acceptable performance is an SDI of less than ± 2.

RIQAS EQA
RIQAS is the world’s largest EQA scheme with more than 75,000 laboratory participants spanning over 138 countries. Choosing an EQA provider is no easy task. That’s why we’ve produce a guide to help you find the right one for you. You can download it here.
At RIQAS, we’re always coming up with new ways to make your performance assessment and result interpretation even easier. We’re also proud of our new programmes and pilot schemes. This year, we’re running pilot programmes for Anti-psychotic drugs, Chagas and Blood Typing.
If you’d like to find out more about the range of programmes we provide, visit our website or download our brochure. Alternatively, you can get in touch with us at marketing@randox.com.
Patient-Centric, Smart Quality Controls for Immunoassays
In 2022, an updated version of ISO15189 was released, placing an emphasis on risk management with the aim of mitigating risk to patients. This updated document means that rigorous quality control (QC) procedures are more important than ever.
ISO15189:2022 cites the use of third-party controls with commutable matrices manufactured to provide concentrations close to clinical decision limits, among others, as crucial considerations. ISO15189:2022 also highlights the importance of identifying and minimising errors in the pre-analytical process. ‘Load & Go’ or ‘Smart’ quality controls are becoming increasingly popular in laboratories around the world to realise this objective.
Smart controls are designed to optimise laboratory workflows, allowing laboratorians to load the control onto an instrument where it can remain until its expiry date, bringing several advantages to laboratories who run immunoassays.
The first is the minimisation of human error and other pre-analytical errors. As these controls are ready-to-go out of the box, there is no chance of reconstitution errors which can result in deviations from target values and contamination which could lead to problematic cross-reactions. Smart quality controls reduce the risk of stability issues resulting from aliquoting or the repetitive opening of vials, and eliminate the possibility of mislabelled controls, while freeing up more storage space.
Smart controls also offer the possibility of improvements in other areas of the laboratory. The reduction in the preparation required for these controls allows laboratories to use this time improving other elements of their QC practices, such as QC analysis and process improvement. Less steps in the QC process not only means time saved in the process itself, but less paperwork for laboratory staff, further freeing up time for more useful practices.
Immunoassay Smart quality controls provide laboratories with an effective QC solution which aids in the optimisation of workflows and the reduction of test turnaround times and the risk of human error throughout the QC process. However, if considering a Smart quality controls for your laboratory, its important to remember the other factors which make a good QC including matrix, stability, and clinically relevant concentrations.
The New Acusera Smart range has been designed to streamline workflows, minimise human error and reduce the strain on your cold storage. The convenient design means these controls can be loaded directly onto the analyser allowing the automation of the QC process, reducing turnaround times and increasing efficiency.
As well as the Immunoassay control, the Acusera Smart range also includes Clinical Chemistry, Liquid Cardiac and Parathyroid Hormone controls. We offer two options: Acusera SmartScan and Acusera SmartLoad. Take a look at the graphic below for more details.

We will be adding more controls to our Smart range soon. To stay up to date with this and all our other product releases, join our mailing list.
If you’d like some more information on any of the products in the Acusera range, don’t hesitate to get in touch. You can contact us at marketing@randox.com.
Meeting Accreditation Guidelines with Acusera 24.7
At Randox Quality Control, we are never finished shouting about how great our interlaboratory comparison and peer group reporting software is. If you’ve had a look yourself, you’ll know exactly why. Acusera 24.7 is full of fetching, interactive charts, and useful, detailed reports, including measurement uncertainty, to help you streamline your QC procedure.
But Acusera 24.7 is so much more than this. Our team are constantly looking for innovative ways to update and improve our live, cloud-based software. Much of this comes from talking to our subscribers and finding out what they want and how they want to do it. Our team also happens to include some serious accreditation enthusiasts. So, we decided to put their passion to work. We’re regularly coming up with new measures to make meeting the guidelines set out by various accreditation bodies, including ISO15189, as simple for you as we can.
In this article, we’ll look at some of the accreditation requirements and the features we’ve included in Acusera 24.7 to simplify the process for you.
QC management tools
Its one thing to look at the features of Acusera 24.7, but what do the various guidelines have to say about QC management tools? Let’s look at some of the major accreditation literature.
ISO15189:2022
The new version of ISO15189 includes updates which aim to place more emphasis on risk management and mitigating risk to the patient. Here’s what the 2022 version has to say about QC management tools:
The Clinical Laboratory Improvement Amendments 1988 (CLIA)
CLIA ’88 regulations are federal standards applicable to all U.S. facilities or sites that test human specimens for health assessment or to diagnose, prevent, or treat disease. These regulations state the following related to QC management:
COLA Accreditation
The Commission on Office Laboratory Accreditation (COLA) is another recognised laboratory accreditation in the U.S. and is a third-party accreditation organisation that ensures laboratories comply with federal regulations, including those set by CLIA. I’m sure you’re catching the trend here:
Meeting accreditation with Acusera 24.7
Acusera 24.7 offers a flexible approach to help laboratories meet all the QC accreditation requirements detailed above, including CLIA, COLA, CAP, and ISO15189.
Our user-friendly, cloud-based software allows users to effortless run statistical analysis including Coefficient of Variation Index (CVI), Standard Deviation Index (SDI), % Bias, Total Error, Sigma Metrics and more! Find out more about how we can aid you in your statistical analysis in our blog, Advanced Statistics with Acusera 24.7.
Acusera 24.7 can also create fully interactive Levey-Jennings charts, and a selection of histograms to provide a wide range of options for the graphical representation of your data. The interactive features of our charts allow you to record events such as lot changes and calibration events directly on to the chart, helping you achieve not just accreditation, but a better understanding of what is going on in your laboratory. You can read more about our charts and the insights you can gain from them at our blog, Charting the course to laboratory excellence.
Acusera 24.7 can also provide you with a variety of reports to help you effortlessly achieve accreditation. From our Statistical Analysis and Exception reports to our Personalised Performance Summary Reports, we can help your laboratory to efficiently identify and document trends or shifts in performance. You can read all about our reports in our blog, Effortless Data Management: Acusera 24.7 Reports.
Measurement Uncertainty
Anyone involved in laboratory quality control will be aware of measurement uncertainty (MU), although that doesn’t mean everyone understands this tricky requirement. MU is defined as a parameter associated with the result of a measurement that characterises the dispersion of values that could reasonably be attributed to the measured quantity.
In other words, MU provides medical laboratories with an estimate of the overall variability in the values they report. The goal of MU is to quantify the doubt or range of possible values around the measurement result, helping to provide an understanding of the reliability and limitations of measurements. This helps ensure measured results are useful and not wildly inaccurate, allows meaningful comparisons with medical decision limits and previous results of the same kind in the same individual and finally, it’s a requirement of ISO15189:2022:
Calculating MU is no simple task and not one that can even be attempted without in depth know-how. These calculations can take a single member of staff 2 full working days to complete. That’s a lot of time away from their normal duties, especially if MU is to be reviewed regularly, as per ISO15189:2022.
Lucky for you, Acusera 24.7 can calculate you MU in seconds, rather than days, and provide you with a report. This report can be shown to your accreditation surveyor, and you can consider the MU box ticked. You can read more about Acusera 24.7 and MU in our Advanced Statistics blog, or in our educational guide How to Measure Uncertainty.
Peer Group Reporting
The peer group reporting features of Acusera 24.7 are much more than just an added extra. Peer group reporting can help speed up the troubleshooting process, allowing you to determine whether an issue you are seeing is unique to you, or evident in the QC data of your peers. It can also provide you with more confidence in assigned target values and help make significant savings by improving your analytical performance, and therefore, your EQA performance.
A peer group reporting programme can also help meet regulatory requirements, like ISO15189:2022:
So, if you’re struggling to find a suitable EQA programme for your analytes, you might just be able to meet your accreditation with the peer group reporting features included in Acusera 24.7.
We’ve only begun to cover the features of this intuitive and efficient software. If you still aren’t convinced that Acusera 24.7 is right for QC data management in your laboratory, reach out to us today at marketing@randox.com. We’re always delighted to hear from you, and we’ll be happy to discuss any of the features of Acusera 24.7, or any reservations you may have.
Our customers can’t believe the gulf in class between Acusera 24.7 and other QC data management programmes.
Don’t get left behind.
Reach out to us today!
UKAS ISO15189:2022 Transition Update
Throughout 2023, UKAS have been hard at work training Assessment Managers and Technical Assessors on the new requirements of the updated ISO15189 guidelines, sharing information about the updated standard and developing the UKAS 15189:2022 Transition Hub providing a one-stop-shop for information on the ISO15189:2022 update.
Recently, UKAS have published a Transition update to remind laboratories of where they stand in seeking their updated accreditation. In this update, UKAS state “As per the UKAS transition plan, all assessments due to take place from the 1st January 2024 will be to ISO15189:2022.”
A gap analysis will be required one month prior to transition assessments, detailing the gaps and the actions which have been taken to remedy these gaps. This should include evidence, such as updated documents and records, embedded in the gap analysis document, showing what action has been taken to bring a laboratory’s practices in line with the updated standard.
An important note included in this transition update is , “UKAS cannot grant accreditation on intent; organisations shall make the necessary changes and have implemented these prior to the transition assessment.” So if your accreditation assessment is due soon, you might want to make use of our ISO15189:2022 Accreditation Guide to assist you in your gap analysis to ensure you don’t miss out.
This is crucial for laboratories because failure to align with the 2022 version of the standard before the deadline of 6th December 2025 will result in a suspension of ISO15189 accreditation for up to 6 months.
Some of the key accreditation updates include:




Randox Quality Control’s Acusera range provides true third part quality controls designed to help you achieve all aspects of ISO15189:2022 accreditation including commutable matrices containing consistent, clinically relevant concentrations with unrivalled consolidation of analytes. To learn more about our range of quality control products, visit our website or, get in touch today at marketing@randox.com
Advanced Statistics with Acusera 24.7
The only thing that sounds more terrifying than statistics, is advanced statistics. For many of us, the dread associated with having to carry out complex calculations can be too much to bear. For others, statistics are not just a set of numbers; they’re a captivating puzzle waiting to be solved. The allure of dissecting intricate patterns, unravelling hidden relationships, and drawing meaningful conclusions makes these statistical enthusiasts embrace the challenges of advanced statistics with excitement rather than apprehension.
No matter which camp you’re in, we bet you’re going to love the advanced statistics features included in Acusera 24.7. From Uncertainty of Measurement to Sigma Metrics, we’ve got you covered. Let’s explore these features and how we can make your statistical analysis easier than ever before.
Measurement Uncertainty
If you’re involved in laboratory quality control, you’ll have heard all about measurement uncertainty (MU). To some it’s intuitive. To some it’s a labyrinth. MU is defined as a parameter associated with the result of a measurement that characterises the dispersion of values that could reasonably be attributed to the measured quantity. For example, if we say the pencil below measures 16cm ± 1cm, at the 95% confidence level we are really saying that we are 95% sure that the pencil measures between 15cm and 17cm.

In other words, the calculation of MU gives medical laboratories an estimate of the overall variability in the values they report. This is important for 3 reasons:
- It helps ensure the measured results are useful and not wildly inaccurate.
- It permits meaningful comparison of medical decision limits and previous results of the same kind in the same individual.
- It’s a regulatory requirement – ISO 15189:2022
All measurements involve some degree of inherent variability due to factors such as instrument limitations, environmental conditions, and biological variation. MU aims to quantify the doubt or range of possible values around the measurement result, helping to provide an understanding of the reliability and limitations of measurements. To complete this task comprehensively, the entire measurement process must be examined and should consider components such as systematic errors, random errors and uncertainties related to calibration, equipment, and the environment.
ISO 15189:2022 states:

So, if you are seeking ISO15189 accreditation, there’s no avoiding MU and advanced statistics. Lucky for you, Acusera 24.7 can calculate MU and provide you with a report which you can export to Excel or PDF for auditing or archiving.
By liberating you from the need to manually calculate MU for all your assays and control levels, Acusera 24.7 streamlines the statistical analysis process, freeing you up to complete your other essential duties. It also helps reduce the chance of errors in the calculation; after all, no matter how talented you are at mathematics, we all make mistakes. The real-time nature of this kind of monitoring means you don’t have to recalculate every time you get more data – simply press the refresh button and you’ll automatically get a new MU report.
By incorporating automated tools to calculate MU, you gain the ability to proactively pinpoint and rectify potential error sources, mitigating the risk of inaccurate measurements and the repercussions that may follow.

For more information on MU and how it’s calculated, see our education guide – How to Measure Uncertainty.
Sigma Metrics
The Sigma model was originally developed for the manufacturing industry as a method of process improvement focusing on minimising errors in process outputs. It has since been adopted by the medical laboratory to improve result reporting.
This model calculates the number of standard deviations or ‘Sigmas’ that fit within the quality specifications of the process – as the sources of error or variation are removed, the standard deviation becomes smaller, and the sigma score increases – 6 being the target. A 6 Sigma process can be expected to produce 3.4 defects, or false results, per million.

Using your predetermined performance limits, including biological variation (standard), RiliBÄK and CLIA, as the total allowable error (TEa), Acusera 24.7 can calculate a Sigma Score for a particular assay, method, or instrument, saving you the hassle of calculating this manually – freeing you up to investigate the sources of error and make improvements to your process.
This is displayed in our Statistical Metrics report along with Count, Bias%, and CV for your chosen range, your cumulative results and those from other Acusera 24.7 users from around the world to provide straightforward and comprehensive statistical analysis and peer group comparison.

Once you’ve found out your Sigma Score for an assay, you can use this to determine your QC frequency and the multi-rules you should apply to your QC. The higher your Sigma Score, the less multi-rules you need to apply to your analysis and the less often you need to run QC for that assay. The table below shows the multi-rules and QC frequencies associated with each Sigma Score.

Acusera 24.7 includes multi-rule capabilities that can be utilised to monitor your QC data and index it as accepted, rejected, or trigger an alert, depending on the pre-defined multi-rules against which you want to check your data. These features enable the identification of nonconformities and reduce the need for laborious manual statistical analysis while enhancing the accuracy and precision of the laboratory. To read more about the multi-rule features of Acusera 24.7, take a look at our educational guide – Understanding QC Multi-rules.

Now that we’ve found which of our assays are underperforming, we can begin to take corrective action. The Sigma Score is affected by bias and imprecision of laboratory results, therefore improving these values will increase the Sigma Score. Some of the steps a laboratory can take are:
- Improved staff training
- Instrument maintenance
- Frequent calibration
- Strict adherence to SOPs when preparing controls and calibrators.
If you are still in the dark ages, carrying out your statistical calculations and analysis manually, reach out to us today to learn more about the time and expense we can help you save. Every day, more people are discovering the power of Acusera 24.7 and the benefits it has in their laboratory.
The updates to ISO151589:2022 are based around increasing patient safety and reducing erroneous results, making advanced statistics essential. Assessors get excited when they see Acusera 24.7 in the lab because they know quitting time is that bit closer. Allow us to help you achieve your accreditation and provide the best possible patient care. With complete onboarding assistance and first-class customer support, you’ll always be ready to get to the bottom of any problems you might face. Get in touch today at marketing@randox.com
Effortless Data Management: Acusera 24.7 Reports
You’ve carried out your daily maintenance and run your IQC. You’ve got your results and now it’s time to type them up into one of your expansive spreadsheets. Reports
You’ve probably got your spreadsheet set up to calculate the required parameters already, but what if there’s an error in the formula? Or what if you make an error when entering your data? Or worse, what if you try to open the spreadsheet only to find that the file is corrupted or lost? If your Excel file is there, someone else might already be editing it, meaning you must wait until they’re finished before you can make any changes.
Even if you face none of these obstacles the labour-intensive statistics needed for performance review and validation might just keep you up at night.
Well, with Acusera 24.7, these concerns are history.
Whether you make use of our automated or semi-automated data entry options, you can be sure that the data put into the system is exactly that returned by your instrument. If you use the manual data entry option, we can’t remove the human error element – but with our simple and intuitive interface, we trust you’ll be flawless anyway. What’s more, the cloud-based nature of our software also means you won’t lose the data by mistake and unique access for each user allows multiple people to be logged in at the same time.

So, what next?
Well, you can view this data on our dashboard for fast and easy access to your results but delve a little deeper into Acusera 24.7 and you can access comprehensive, easy-to-read, customisable, reports designed to speed up the review process.
These reports include statistical analysis, exception reports, peer group statistics, uncertainty of measurement and advanced statistical metrics. The latter two we’ll look at in a dedicated article. For the others, however, let’s dive in and see how you could benefit from our range of extensive reports.
Statistical Analysis Reports
The first report we will look at is the statistical analysis report. This report allows you to view your IQC data from a specified date range, and compare it to your cumulative data, that is, all the IQC data you’ve collected since you began using that lot, as well as the peer group data for the same lot all within one screen. If you are part of a chain of laboratories, you can compare this data with your laboratory group to see how your lab stacks up by using the World/Group toggle button.
This report provides you with the count, mean, SD, CV, SDI and CVI for a lot and can be organised by assay, as shown in the image below, instrument, or method, allowing you full freedom to customise this report to suit your needs. Don’t forget, like all our reports and charts, this data is fully exportable to PDF or Excel for filing or data review.
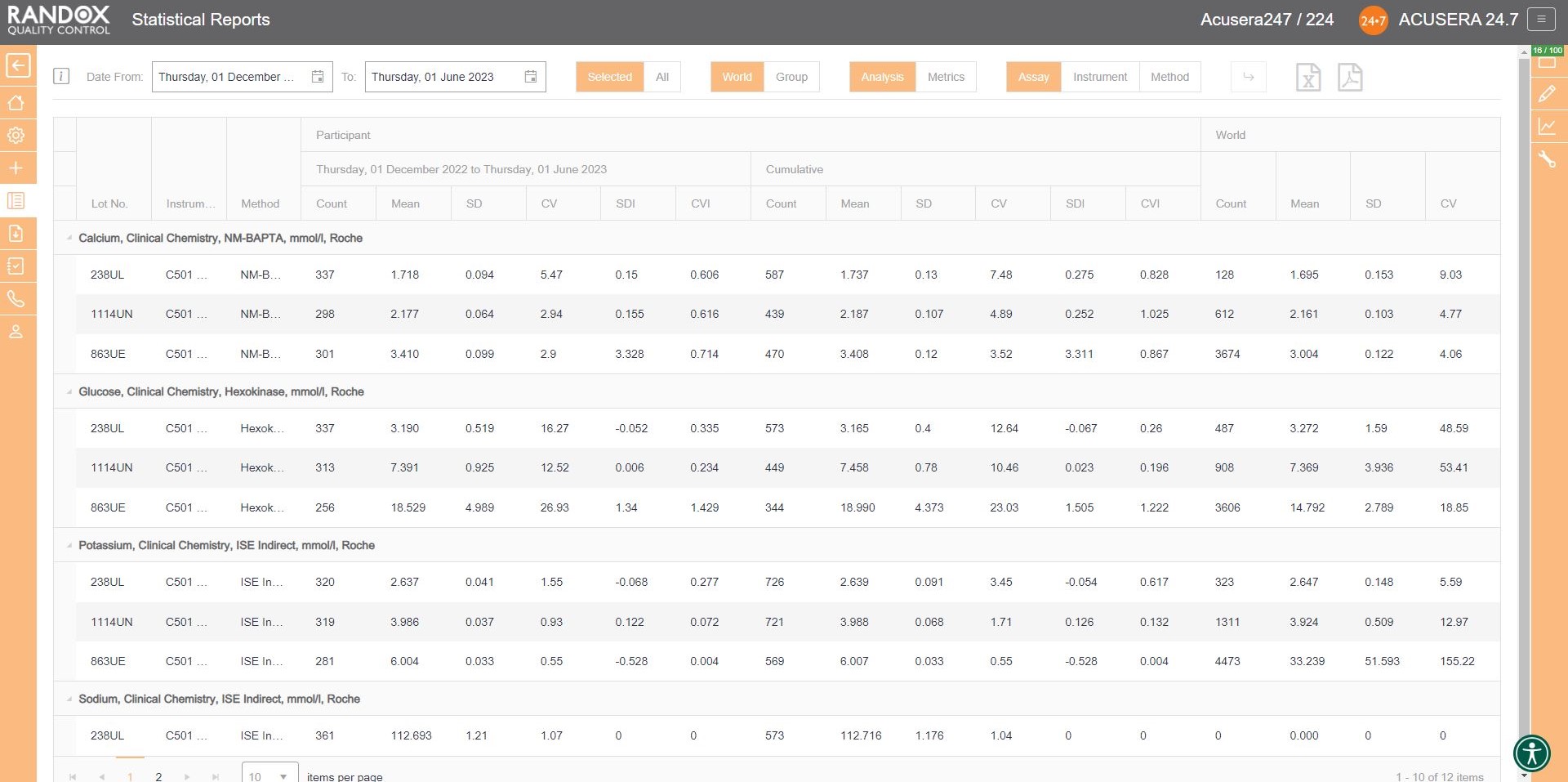
Handy, right? This report provides you with everything you need to carry out the validation and verification of new IQC lots, plus much more. We’ll look at this in more detail in an upcoming article.
Exception Reports
If you wish to determine your best and worst-performing tests, our exception report is perfect for you. This report is designed to quickly and easily identify assays with a high percentage of errors. The exception report provides an on-screen summary of the number of QC results for each individual assay and control lot that fall within the following categories: <2SD, 2-3SD and >3SD. This comprehensive performance review can be filtered: by clicking on the top of the ‘>3DSI’ column, this report will display assays in descending order with your worst-performing assays at the top, as shown below.
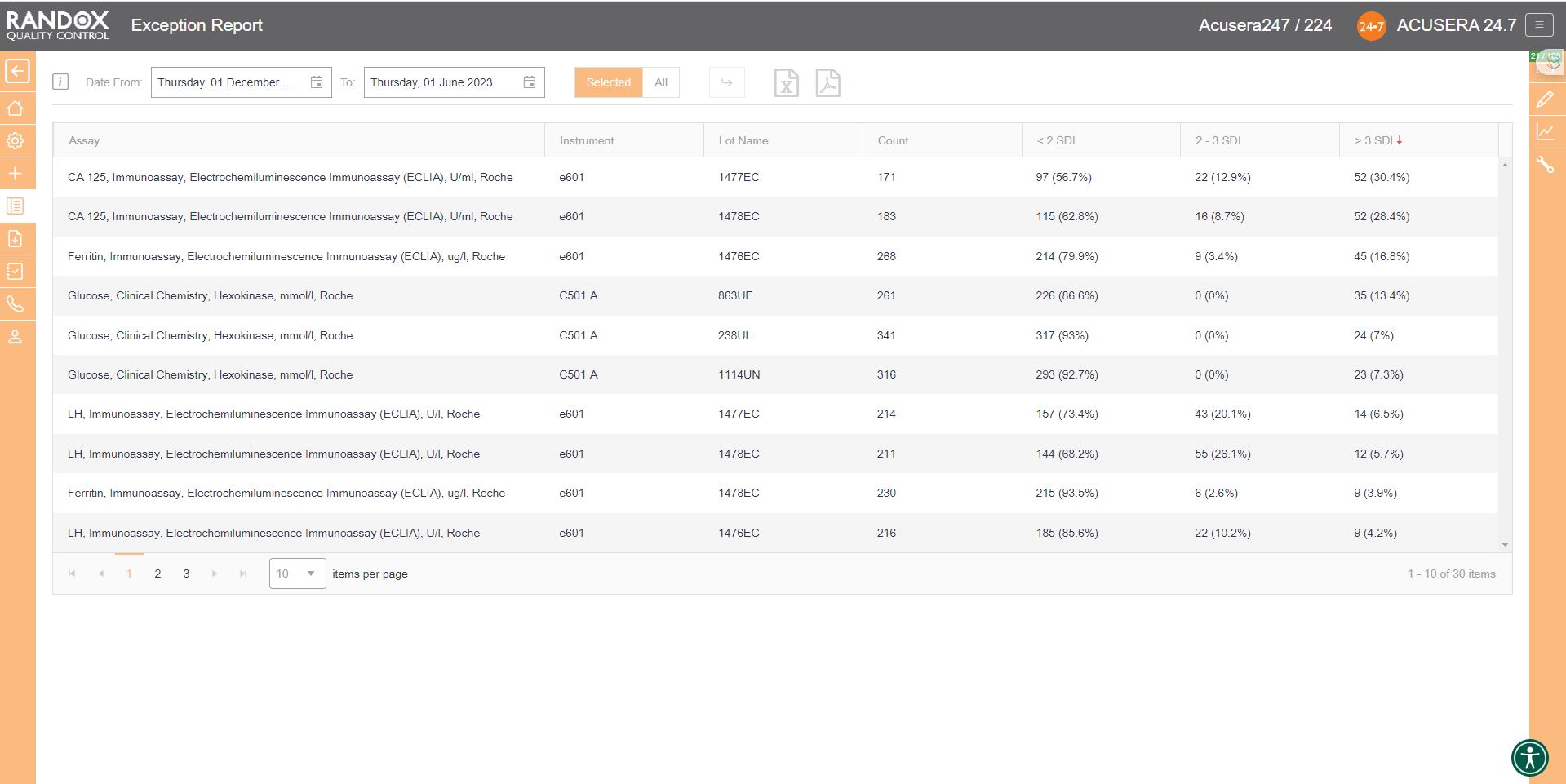
Filtering by ‘<2SDI’, it will display the same data with your best-performing assays at the top.
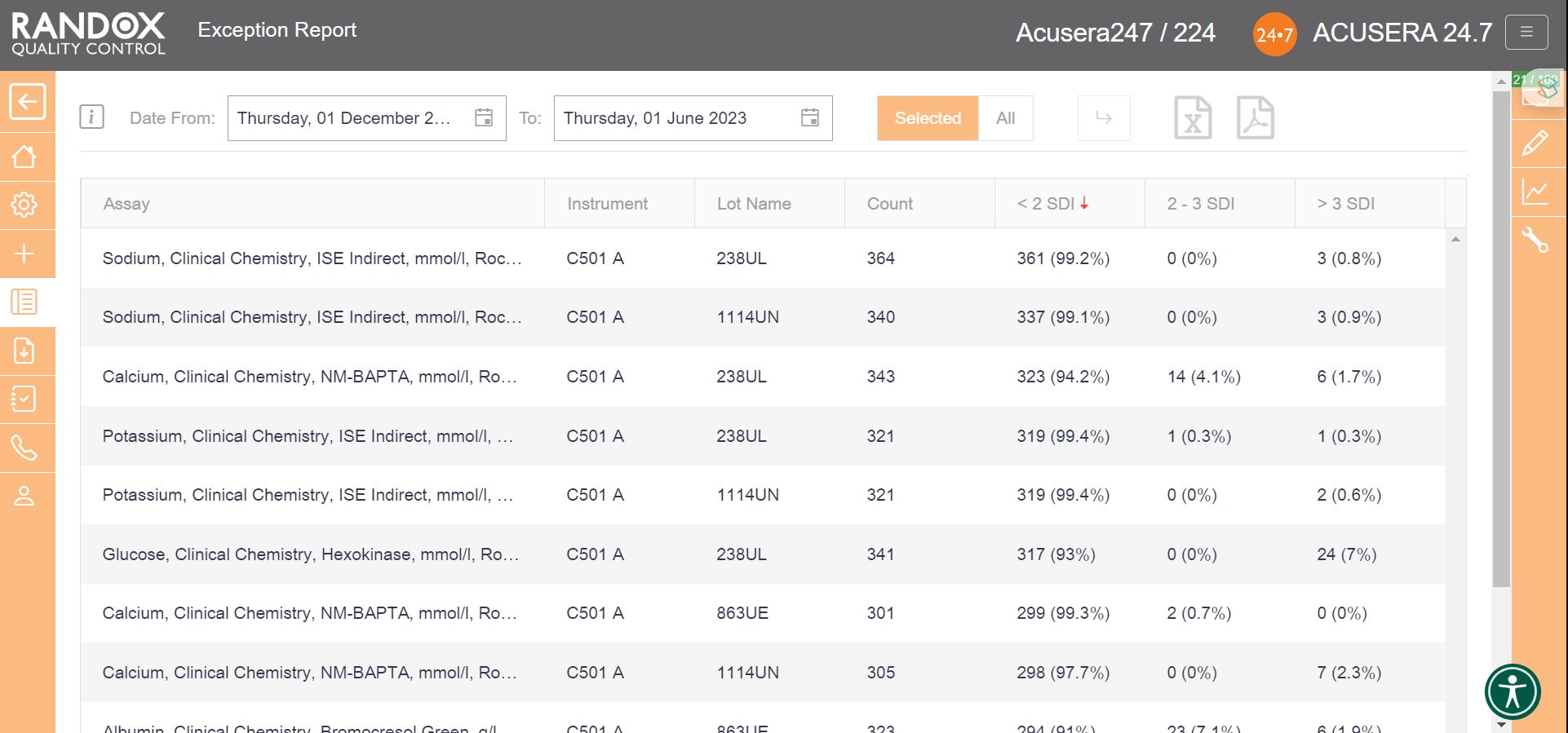
With this information, you can determine in which of your assay’s failures most often occur and encourage staff to look a little more closely at why failures arise and identify changes to improve and minimise errors.
Peer Group Statistics Reports
Now that you have figured out the performance of your assays, you’ll want to see how you compare with others running the same tests. Our Peer Group Statistics Report is your new best friend.
Updated live and in real-time, with no submission deadlines, you can compare your statistics to those of your peer group, determined by analyte, method, instrument manufacturer and model.
Simply select the IQC lot you wish to analyse and Acusera 24.7 will generate the data for you, displaying the count of QC data, mean, SD and CV, giving you comprehensive insight into your performance vs your peers.
You can customise this report even further. If you select an analyte, we’ll show you the data for that analyte alone. If not, we’ll show you the information for all analytes related to that lot. The same goes for specifying a date range – if you choose a range, we’ll show you the data inside that range alone. If not, we’ll show you all the data for your chosen lot.
By clicking on the headers, you can filter the data – 1 click will display the data in ascending order, 2 will show you a list in descending order and 3 clicks will reset the table.
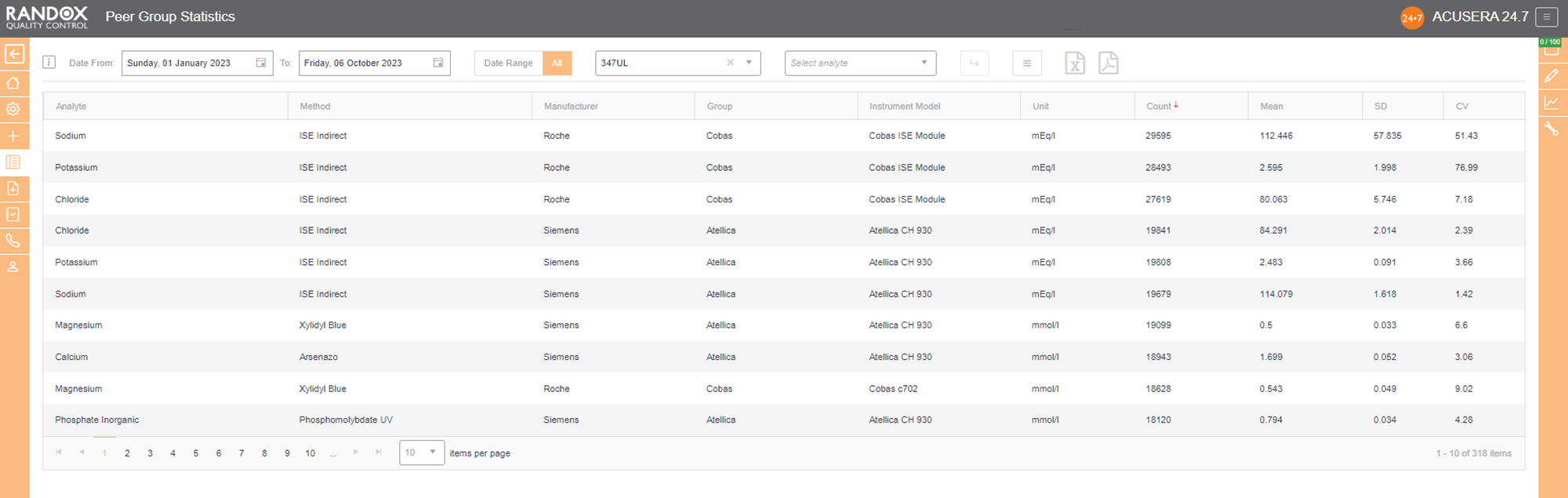
When these reports are combined with the other impressive features of Acusera 24.7, like our fully customisable charts or advanced statistical analysis, this software can help streamline your IQC procedure and data review process.
When the accreditors come knocking, others will be scuffling around trying to gather multitudes of reports and files, but you will be sitting with a smile on your face and your feet up, because you’ve got Acusera 24.7.
With full onboarding assistance and technical support that’s top-of-the-class, you’ll always have someone to help you get to the bottom of any problems that you face.
If you haven’t already booked a demo, get in touch with us today and let us show you how much time we can save you with this innovative and intuitive software. Alternatively, take a look at our Resource Hub for some material on Acusera 24.7 or Acusera IQC.
To streamline your QC Data analysis, get in touch with us at marketing@randox.com.











