Effortless Data Management: Acusera 24.7 Reports
Effortless Data Management: Acusera 24.7 Reports
You’ve carried out your daily maintenance and run your IQC. You’ve got your results and now it’s time to type them up into one of your expansive spreadsheets. Reports
You’ve probably got your spreadsheet set up to calculate the required parameters already, but what if there’s an error in the formula? Or what if you make an error when entering your data? Or worse, what if you try to open the spreadsheet only to find that the file is corrupted or lost? If your Excel file is there, someone else might already be editing it, meaning you must wait until they’re finished before you can make any changes.
Even if you face none of these obstacles the labour-intensive statistics needed for performance review and validation might just keep you up at night.
Well, with Acusera 24.7, these concerns are history.
Whether you make use of our automated or semi-automated data entry options, you can be sure that the data put into the system is exactly that returned by your instrument. If you use the manual data entry option, we can’t remove the human error element – but with our simple and intuitive interface, we trust you’ll be flawless anyway. What’s more, the cloud-based nature of our software also means you won’t lose the data by mistake and unique access for each user allows multiple people to be logged in at the same time.

So, what next?
Well, you can view this data on our dashboard for fast and easy access to your results but delve a little deeper into Acusera 24.7 and you can access comprehensive, easy-to-read, customisable, reports designed to speed up the review process.
These reports include statistical analysis, exception reports, peer group statistics, uncertainty of measurement and advanced statistical metrics. The latter two we’ll look at in a dedicated article. For the others, however, let’s dive in and see how you could benefit from our range of extensive reports.
Statistical Analysis Reports
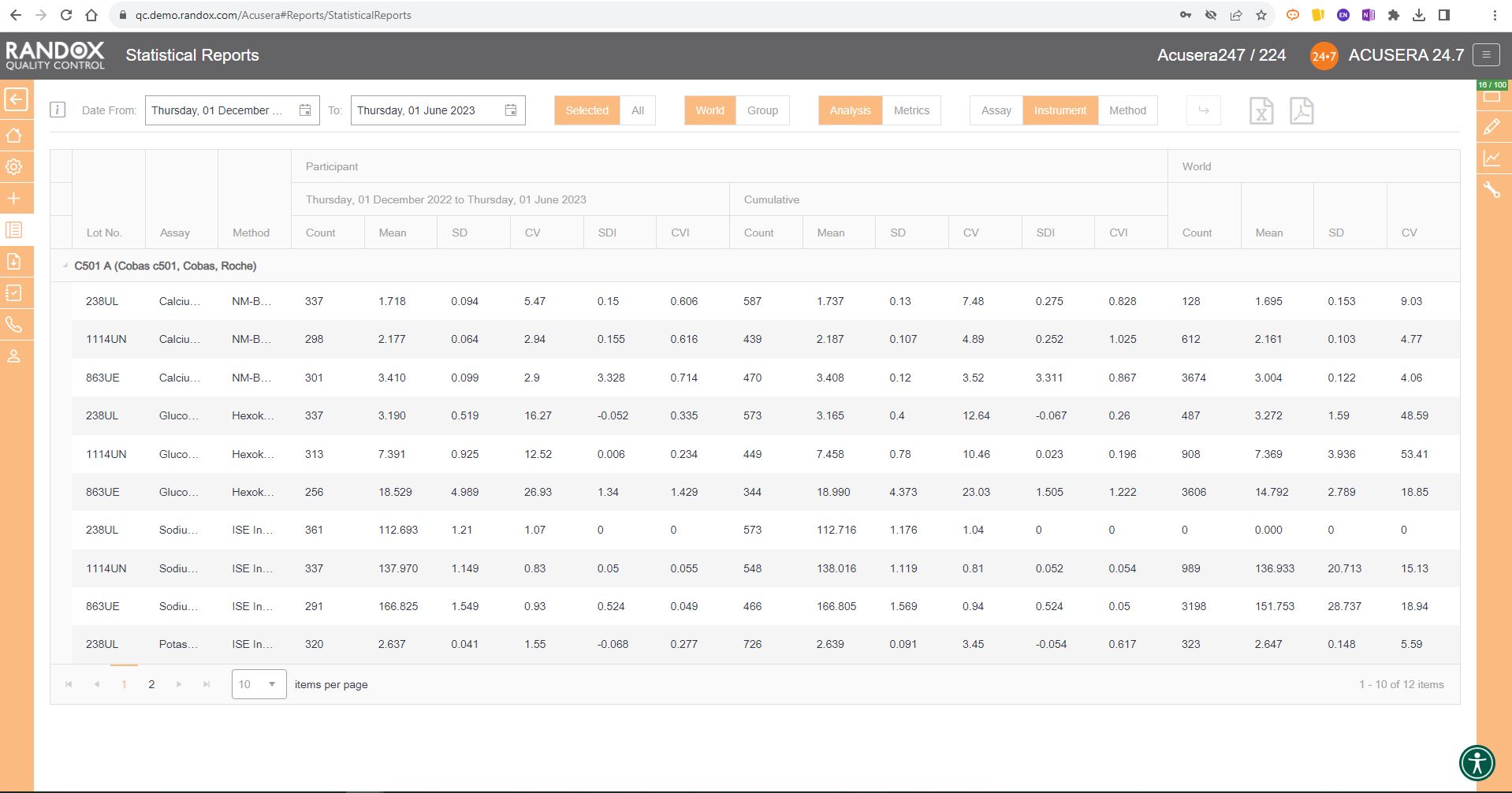
The first report we will look at is the statistical analysis report. This report allows you to view your IQC data from a specified date range, and compare it to your cumulative data, that is, all the IQC data you’ve collected since you began using that lot, as well as the peer group data for the same lot all within one screen. If you are part of a chain of laboratories, you can compare this data with your laboratory group to see how your lab stacks up by using the World/Group toggle button.
This report provides you with the count, mean, SD, CV, SDI and CVI for a lot and can be organised by assay, as shown in the image below, instrument, or method, allowing you full freedom to customise this report to suit your needs. Don’t forget, like all our reports and charts, this data is fully exportable to PDF or Excel for filing or data review.
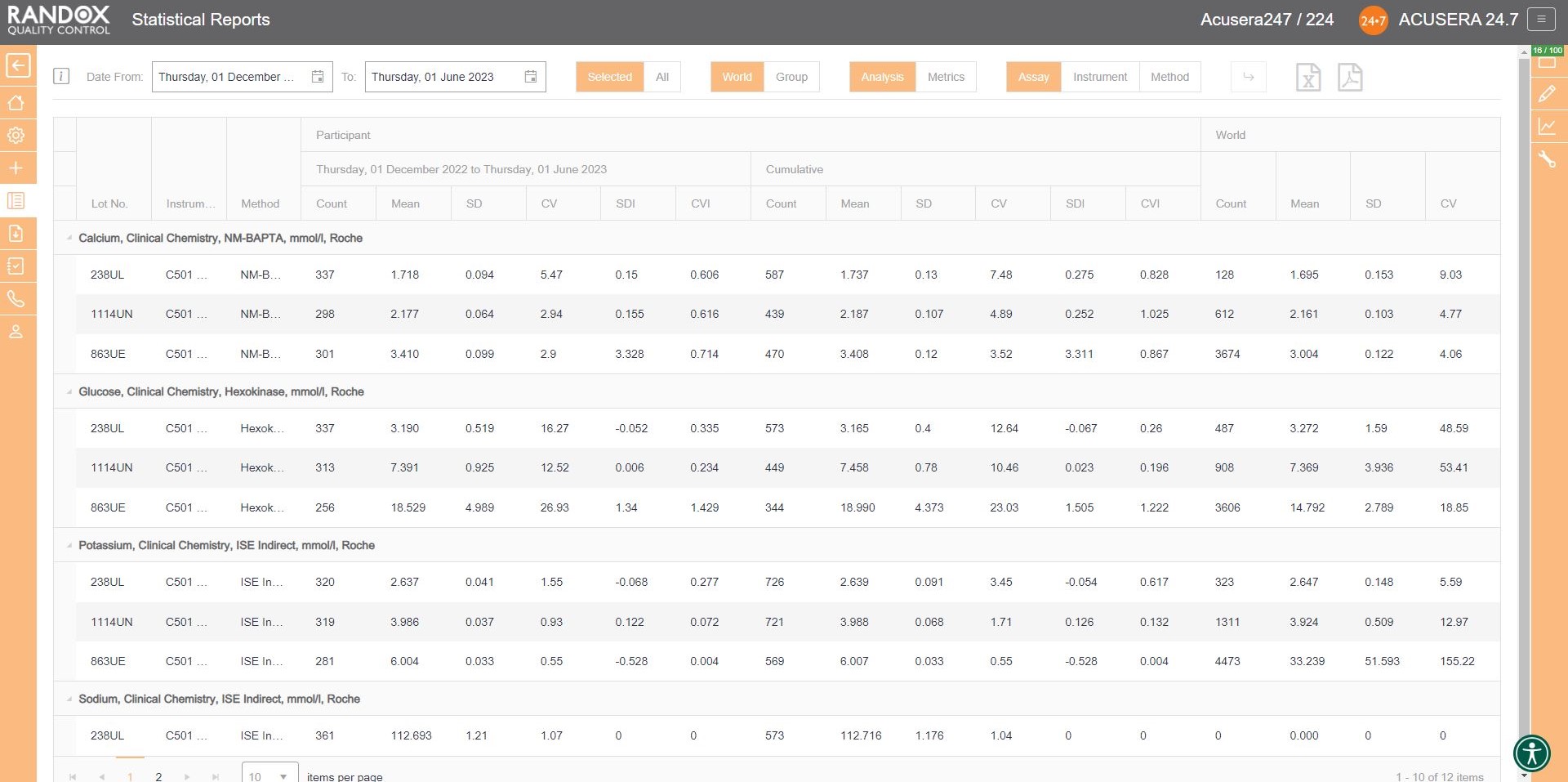
Handy, right? This report provides you with everything you need to carry out the validation and verification of new IQC lots, plus much more. We’ll look at this in more detail in an upcoming article.
Exception Reports
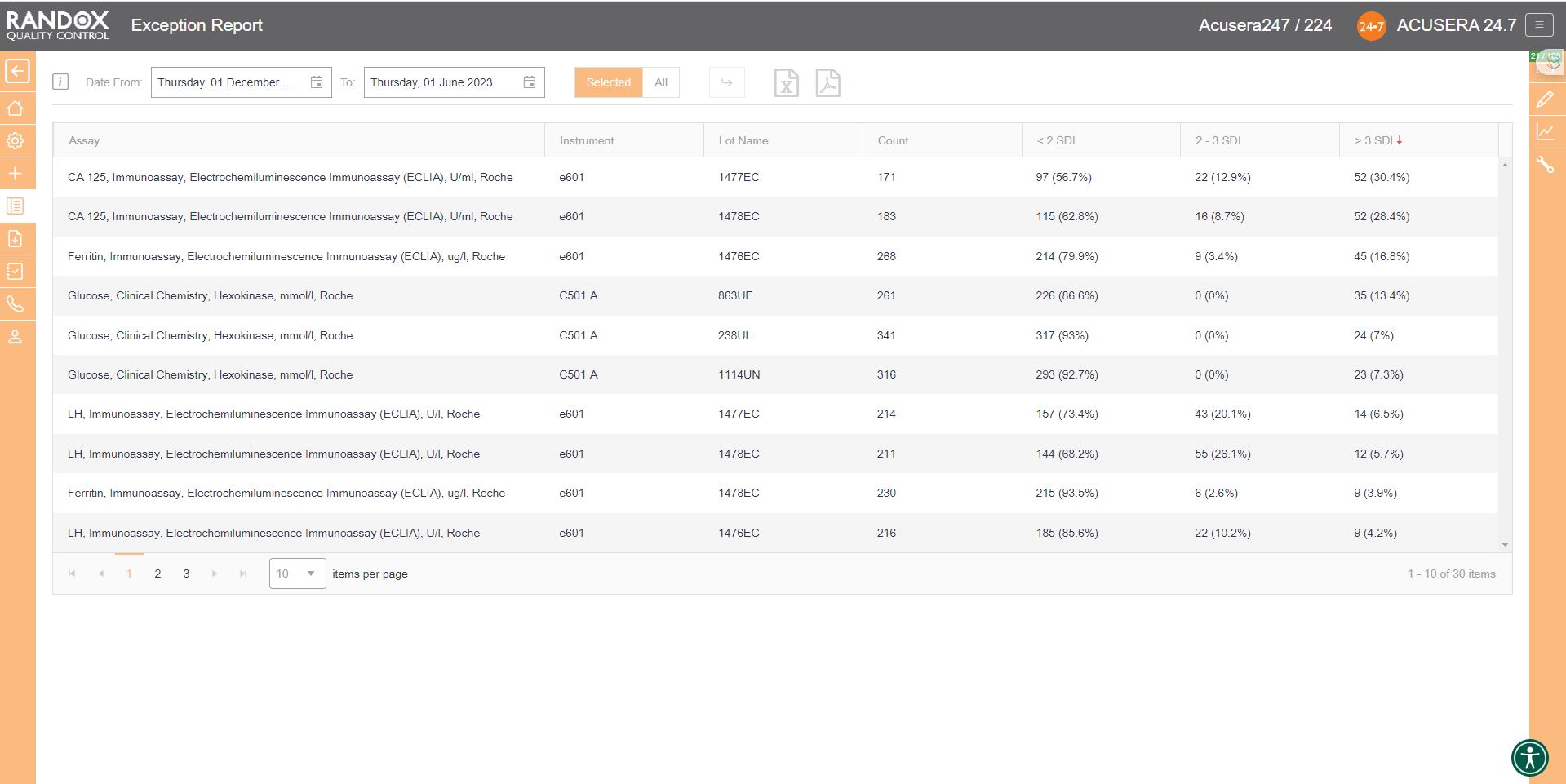
If you wish to determine your best and worst-performing tests, our exception report is perfect for you. This report is designed to quickly and easily identify assays with a high percentage of errors. The exception report provides an on-screen summary of the number of QC results for each individual assay and control lot that fall within the following categories: <2SD, 2-3SD and >3SD. This comprehensive performance review can be filtered: by clicking on the top of the ‘>3DSI’ column, this report will display assays in descending order with your worst-performing assays at the top, as shown below.
Filtering by ‘<2SDI’, it will display the same data with your best-performing assays at the top.
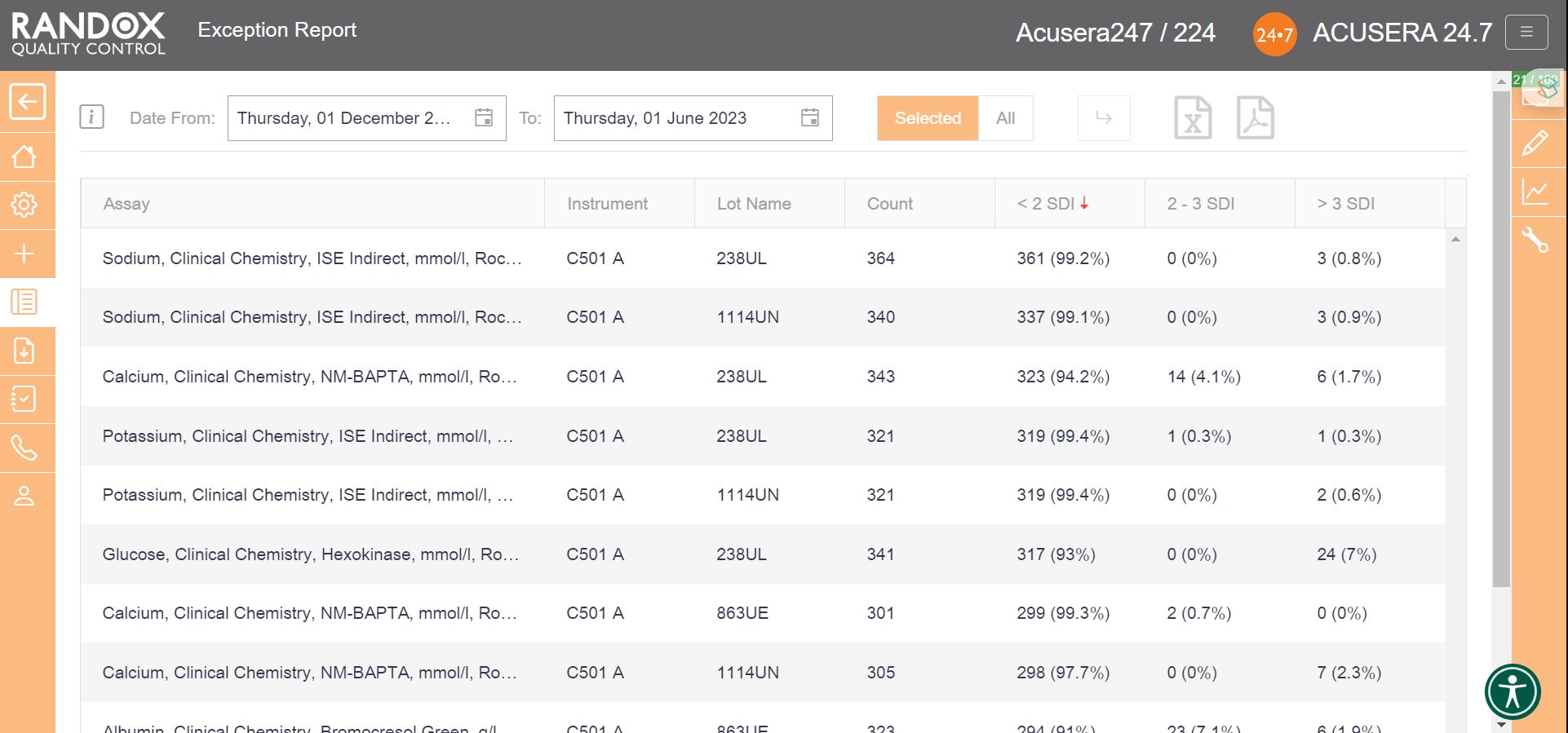
With this information, you can determine in which of your assay’s failures most often occur and encourage staff to look a little more closely at why failures arise and identify changes to improve and minimise errors.
Peer Group Statistics Reports
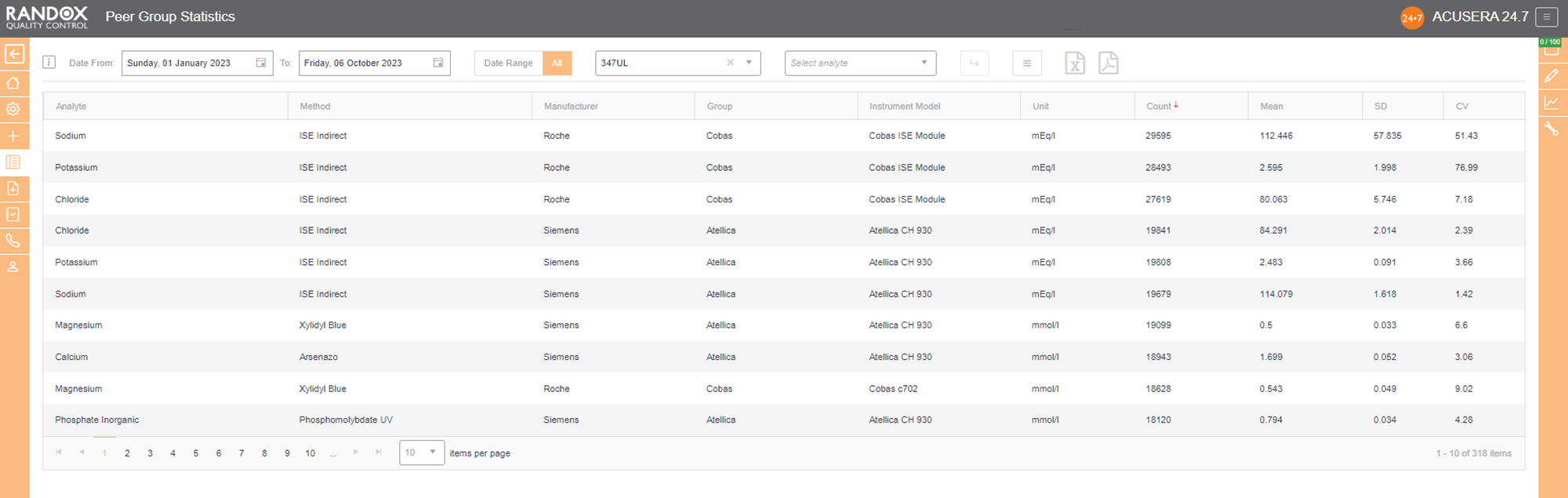
Now that you have figured out the performance of your assays, you’ll want to see how you compare with others running the same tests. Our Peer Group Statistics Report is your new best friend.
Updated live and in real-time, with no submission deadlines, you can compare your statistics to those of your peer group, determined by analyte, method, instrument manufacturer and model.
Simply select the IQC lot you wish to analyse and Acusera 24.7 will generate the data for you, displaying the count of QC data, mean, SD and CV, giving you comprehensive insight into your performance vs your peers.
You can customise this report even further. If you select an analyte, we’ll show you the data for that analyte alone. If not, we’ll show you the information for all analytes related to that lot. The same goes for specifying a date range – if you choose a range, we’ll show you the data inside that range alone. If not, we’ll show you all the data for your chosen lot.
By clicking on the headers, you can filter the data – 1 click will display the data in ascending order, 2 will show you a list in descending order and 3 clicks will reset the table.
When these reports are combined with the other impressive features of Acusera 24.7, like our fully customisable charts or advanced statistical analysis, this software can help streamline your IQC procedure and data review process.
When the accreditors come knocking, others will be scuffling around trying to gather multitudes of reports and files, but you will be sitting with a smile on your face and your feet up, because you’ve got Acusera 24.7.
With full onboarding assistance and technical support that’s top-of-the-class, you’ll always have someone to help you get to the bottom of any problems that you face.
If you haven’t already booked a demo, get in touch with us today and let us show you how much time we can save you with this innovative and intuitive software. Alternatively, take a look at our Resource Hub for some material on Acusera 24.7 or Acusera IQC.
To streamline your QC Data analysis, get in touch with us at marketing@randox.com.
Charting the Course to Laboratory Excellence
Are you still using spreadsheets for your QC data and Charts?
You’ve been left behind.
But don’t worry!
Your laboratory’s ultimate ally in the quest for precision and excellence has arrived.
Acusera 24.7 is a tool that not only streamlines your QC data but also empowers you with a treasure trove of invaluable charts.
These charts are more than just numbers and lines; they are your secret weapon for troubleshooting, achieving accreditation, and driving continuous process improvement.
Acusera 24.7 doesn’t just offer charts. It offers a symphony of insights at your fingertips. From the precision of interactive Levey-Jennings charts to the competitive edge of performance summary charts for peer group comparison, from the rhythm of weekly mean charts to the clarity of reliable SD histograms – these charts are your compass in the world of quality control.
The best part?
You’re in control.
Tailor these charts to your unique needs, whether you’re dealing with single or multiple analytes, an abundance of QC lots, fixed or variable SDs, or need to pinpoint data within a specific date range.
Join us on a journey through the world of Acusera 24.7’s charts, where data becomes your strategic advantage, and discover why more laboratories are choosing Acusera 24.7 for QC data management every day.
Levey-Jennings Charts
Every laboratorian has seen countless Levey-Jennings charts and for good reason.
These charts are the unsung heroes of quality control in the laboratory.
They offer a visual snapshot of data over time, helping to detect trends, outliers, and systematic errors that might otherwise go unnoticed. Levey-Jennings charts are like the heartbeat monitor of your laboratory, providing real-time insights into the health of your analytical processes.
We’ve taken Levey-Jennings charts to the next level.
Our colourful graphs might look like they belong in a modern art museum, but trust me, they’re more than just eye candy.
Acusera 24.7’s Levey-Jennings charts are like the laboratory’s personal detective, sniffing out anomalies and shifts and making sure your QC data behaves.
Let’s have a look at what you can do with the Acusera 24.7 interactive Levey-Jennings charts.
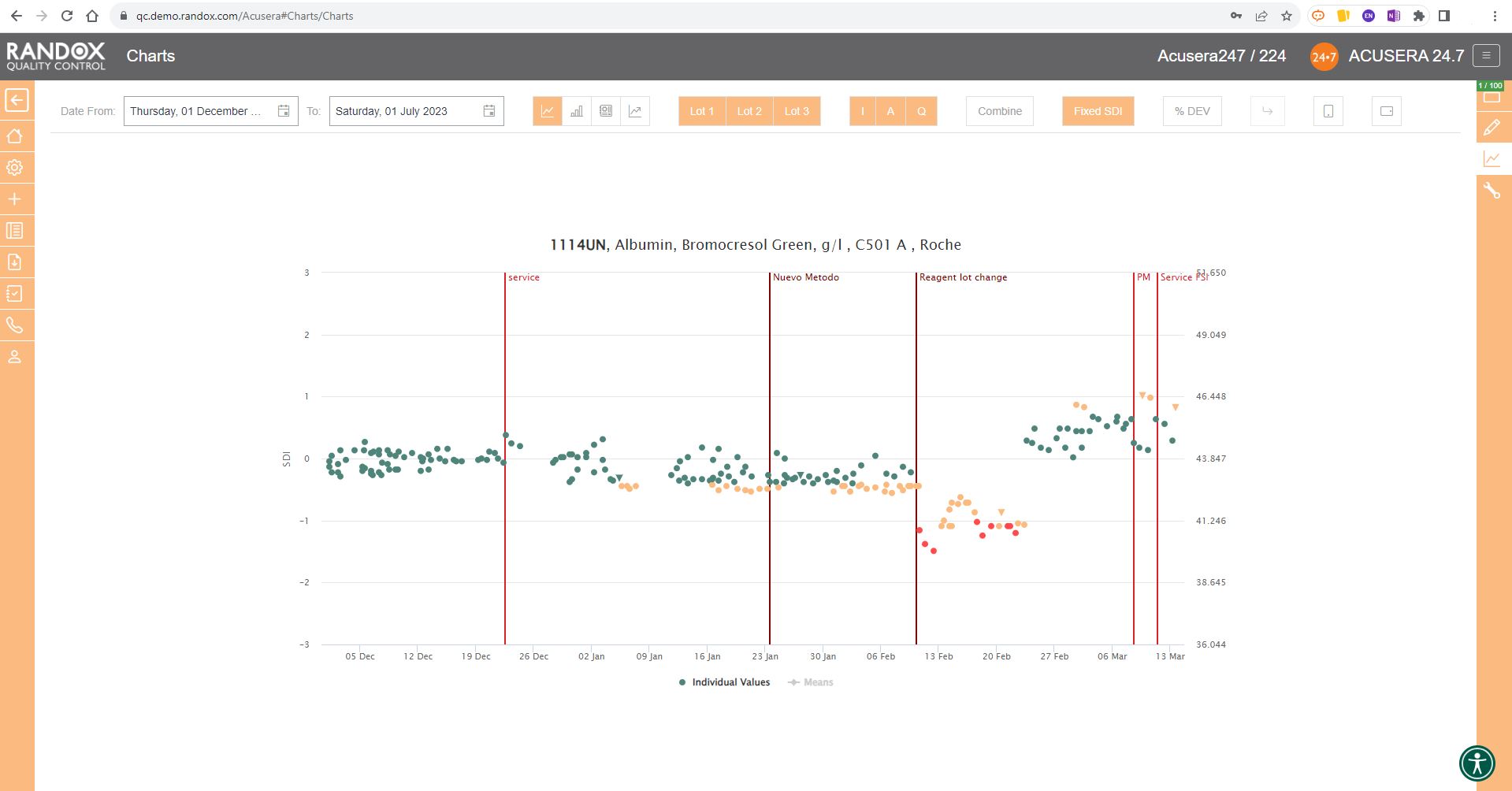
The screenshot below shows a Levey-Jennings chart for a single analyte, with the date on the X-axis and SD on the Y-axis. On this chart, you can see data points displayed in different colours. Green data points indicate an acceptable result. Orange points show data that has triggered your predefined alert criteria, while red points are those that have broken your set rejection rules.
The lines marked on the chart below represent events that have been recorded. Instrument events such as calibration events or maintenance can be recorded to monitor their effects on your QC, allowing you to quickly see how these events relate to any deviations or improvements in your QC data. For example, after the event labelled ‘Reagent lot change’ you can see a series of alerts and failures. Marking this event on the chart allows for an at-a-glance explanation of this deviation. These events are completely customisable so you can record any relevant information you want!
Finally, data points that appear as a triangle indicate a comment has been added. What text is included in the comment is completely up to you!
The next screenshot below shows a Levey-Jennings chart containing QC data for all the tests included in the Clinical Chemistry Panel.
Acusera 24.7 panels allow you to group related tests together, helping increase the efficiency of your data review.
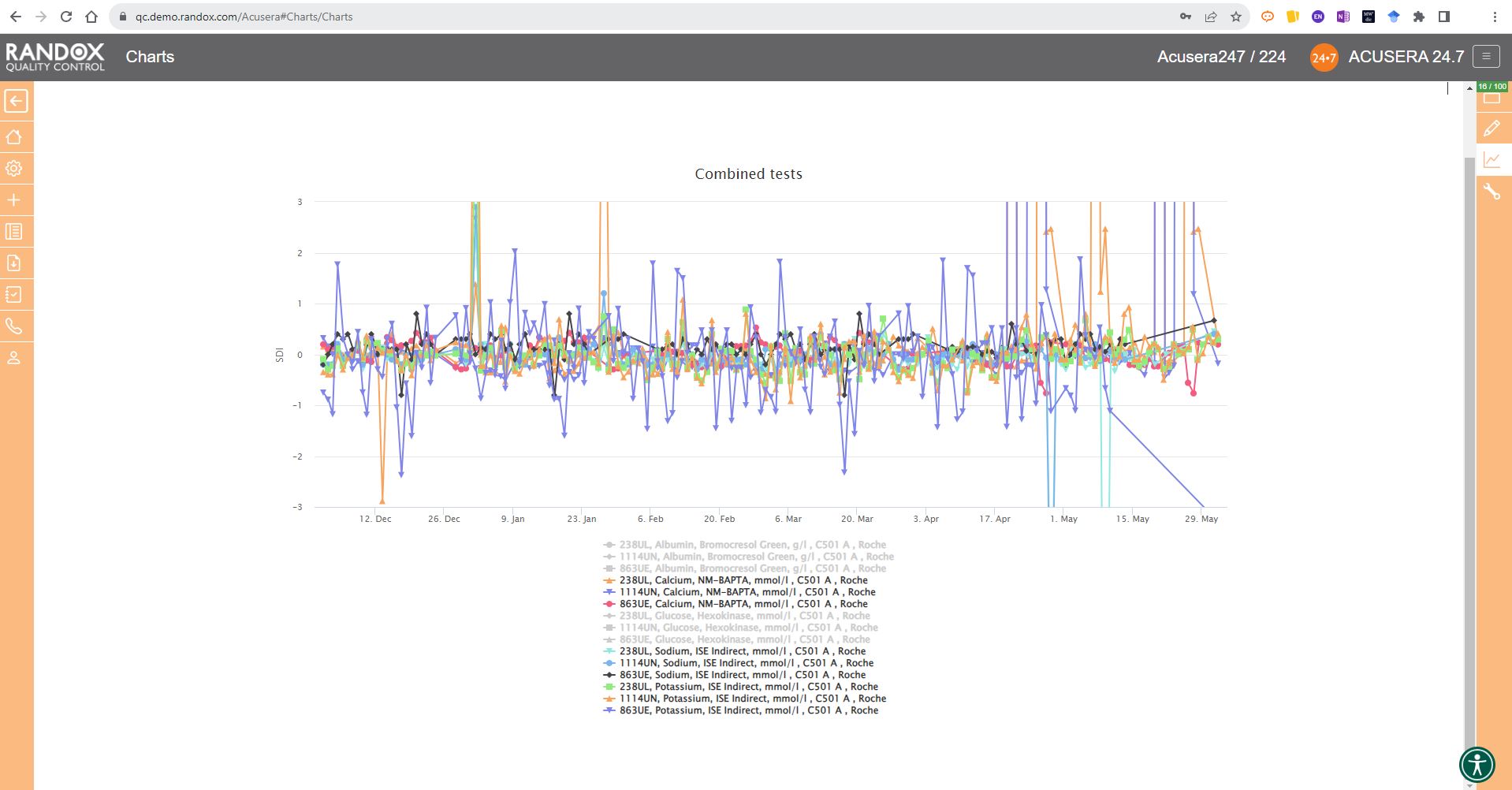
It looks great, right?
Maybe a little confusing.
The screenshot is perhaps a little deceptive.
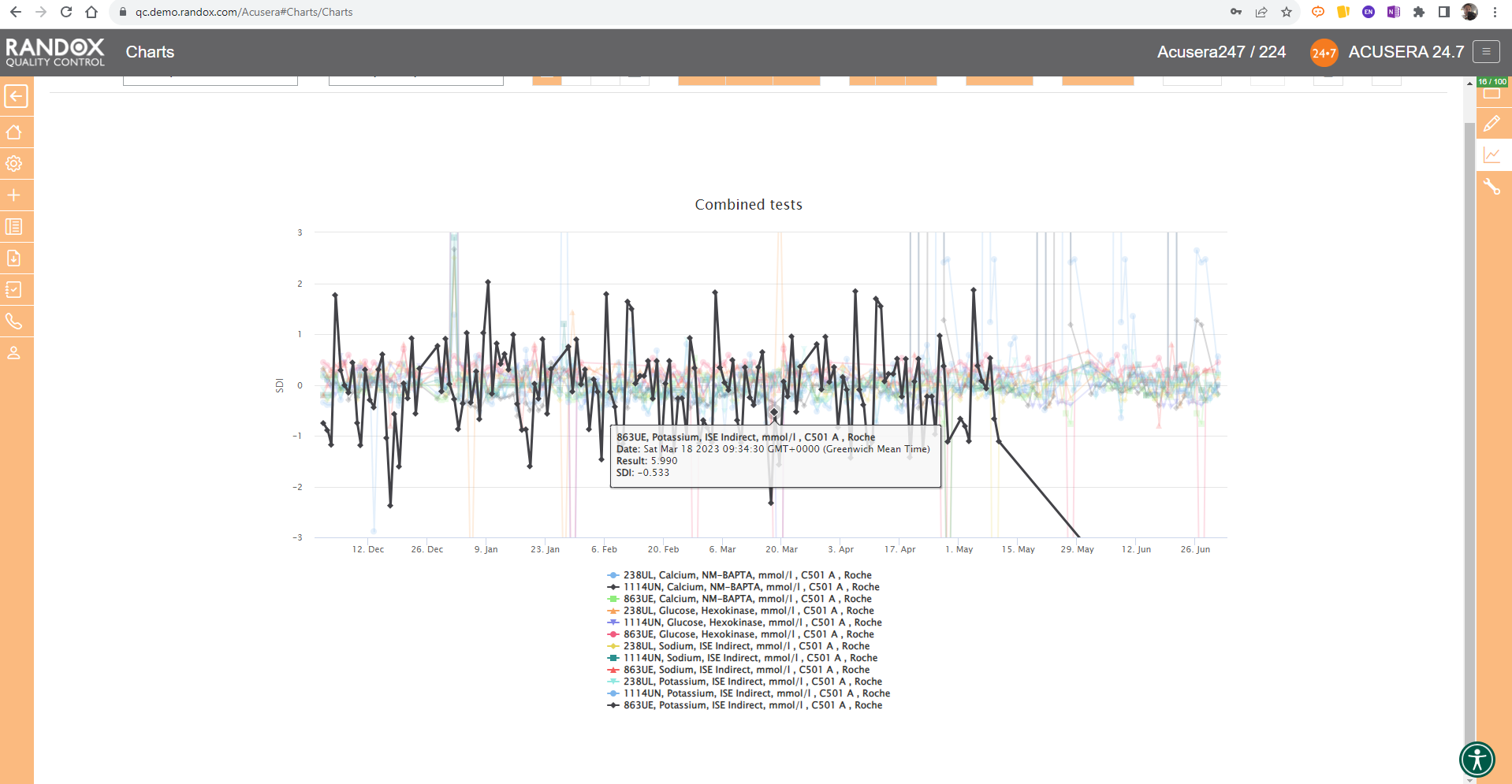
When viewing these charts live, you can view the data as a whole, or home in on individual data sets by simply hovering over the data you want to see. You can also selected a deselect datasets at will by clicking on its name in the list below the chart.
The screenshot below shows an example of this.
All the charts we’ve looked at so far have had a fixed 3SD on the Y-axis.
For a more in-depth review of your data, you may wish to expand this axis.
With the click of a button, you can expand the Y-axis to include all your data points. See below for an example.

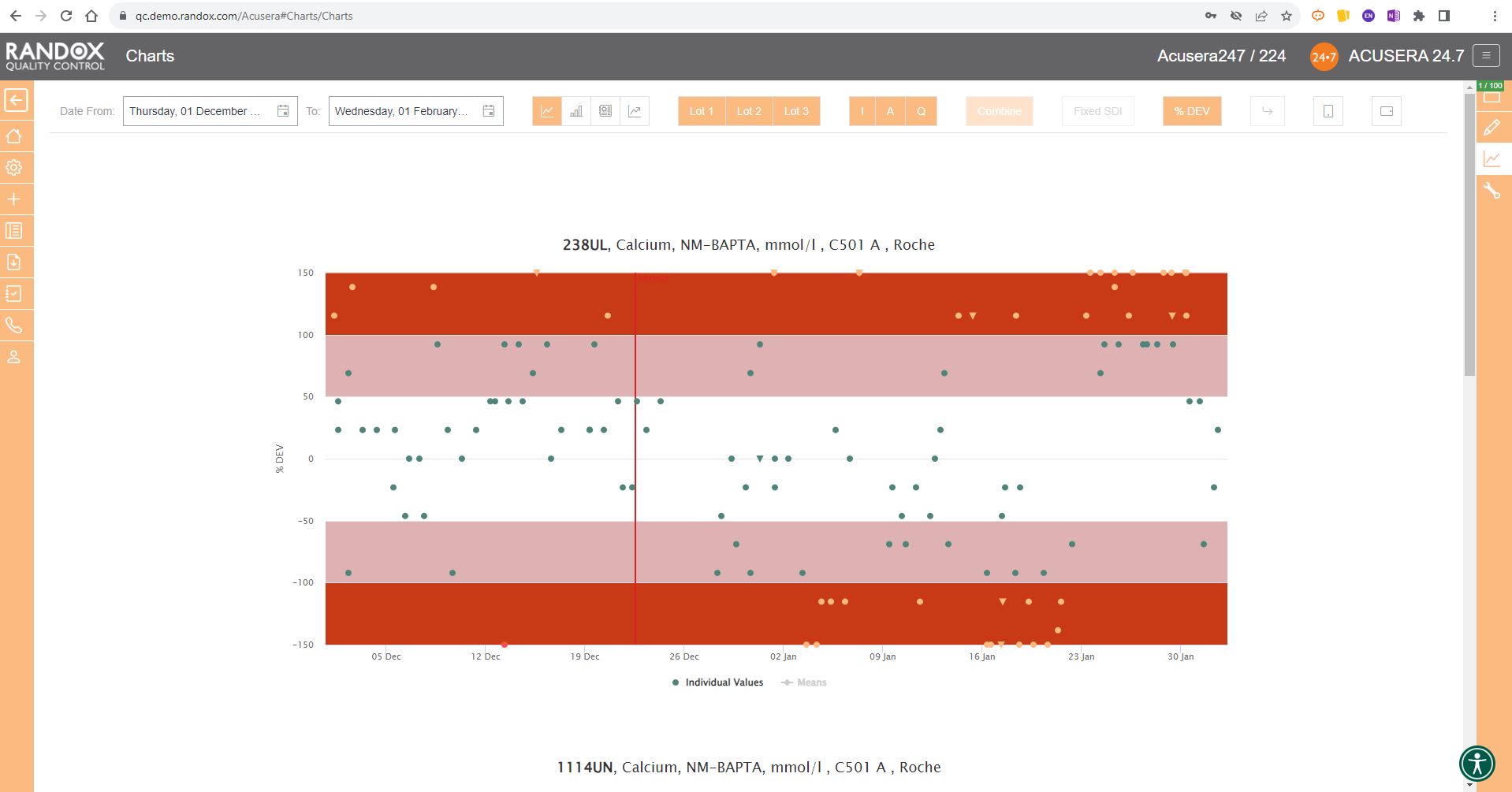
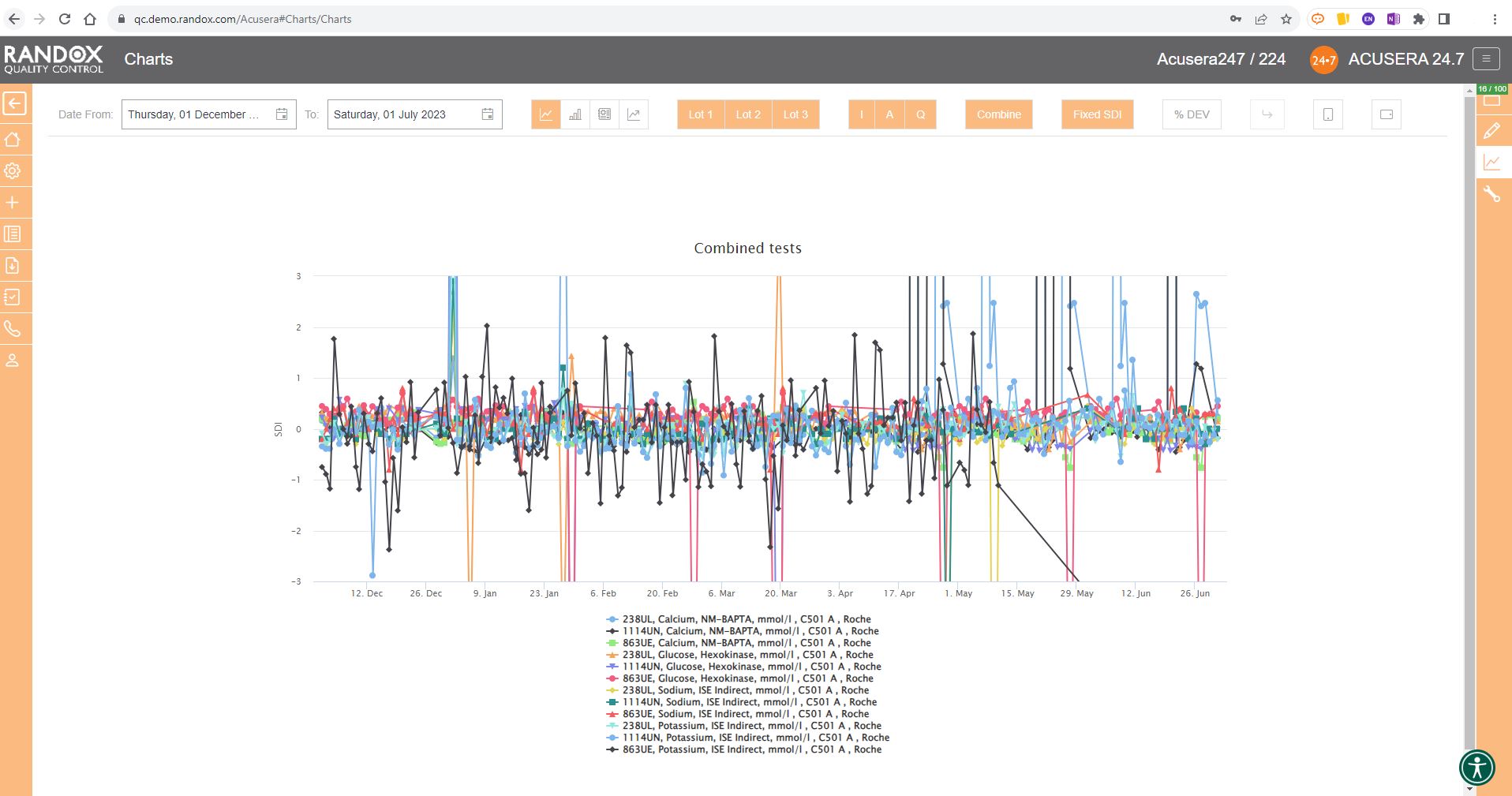
In some cases, you may wish to view this data displayed as ‘% Deviation’.
Again, with the click of a single button, you can convert the Y-axis to show just that, as shown below.
Performance Summary Charts
Peer group comparison of IQC data has a lot of benefits.
Comparing your data with other laboratories that use the same QC lot, instrument, method and more, can help you with troubleshooting and continuous process improvement.
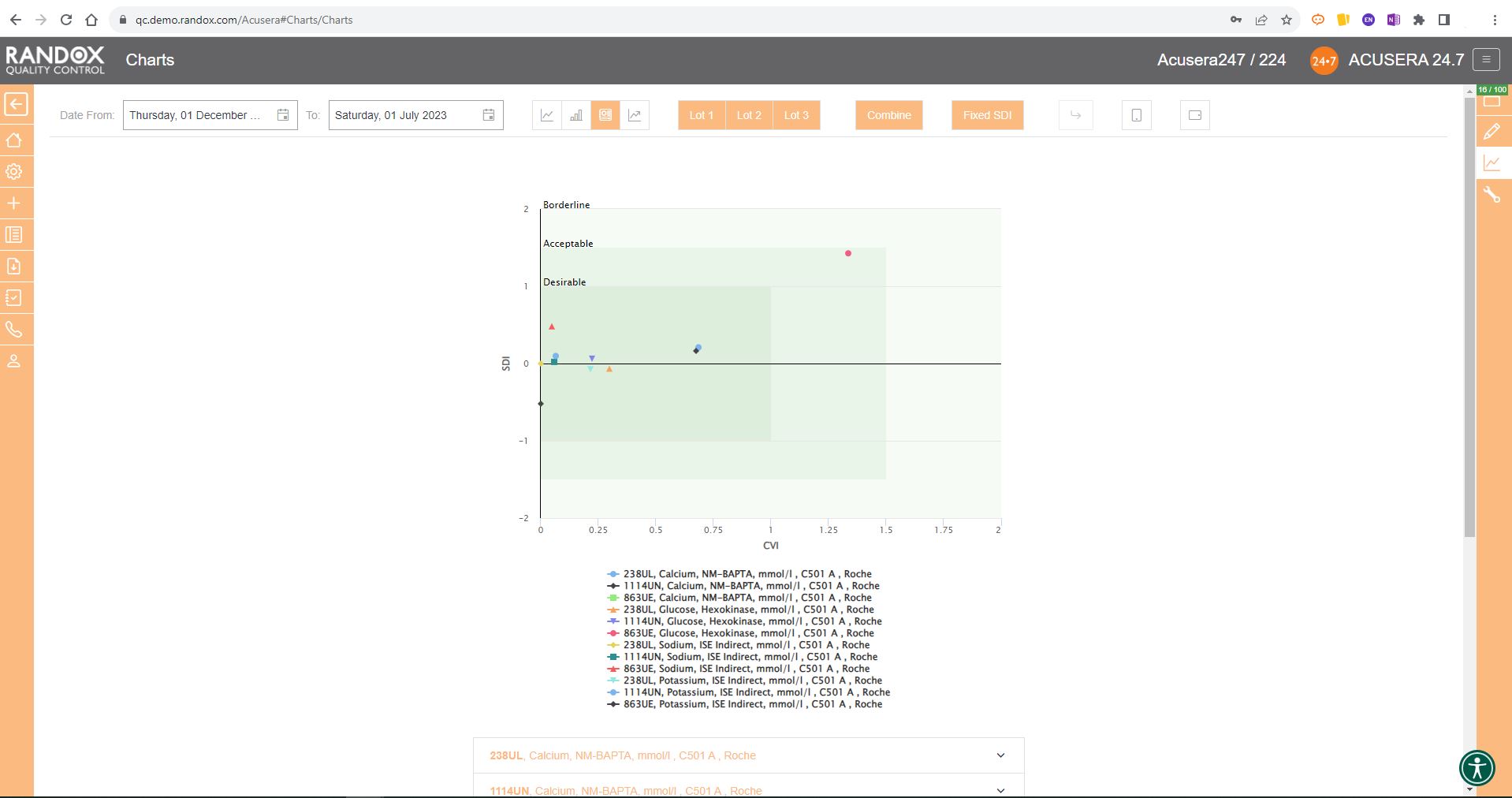
The Acusera 24.7 Performance Summary Charts do all the work for you.
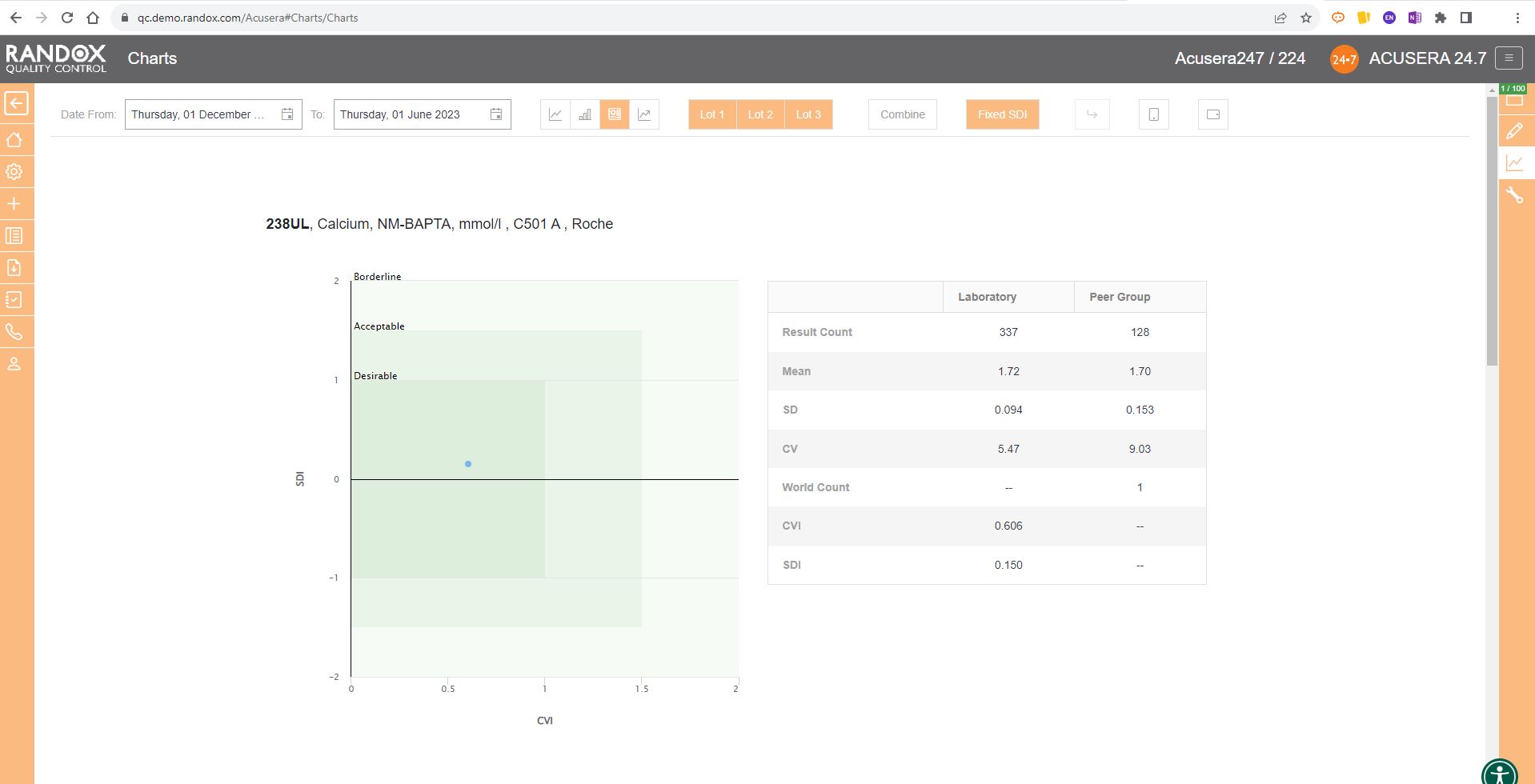
As shown in the screenshot below, these charts display your data and how it compares to your peers including mean, CV, and SD.
You can also view this data in a table to get a more detailed picture of your performance.
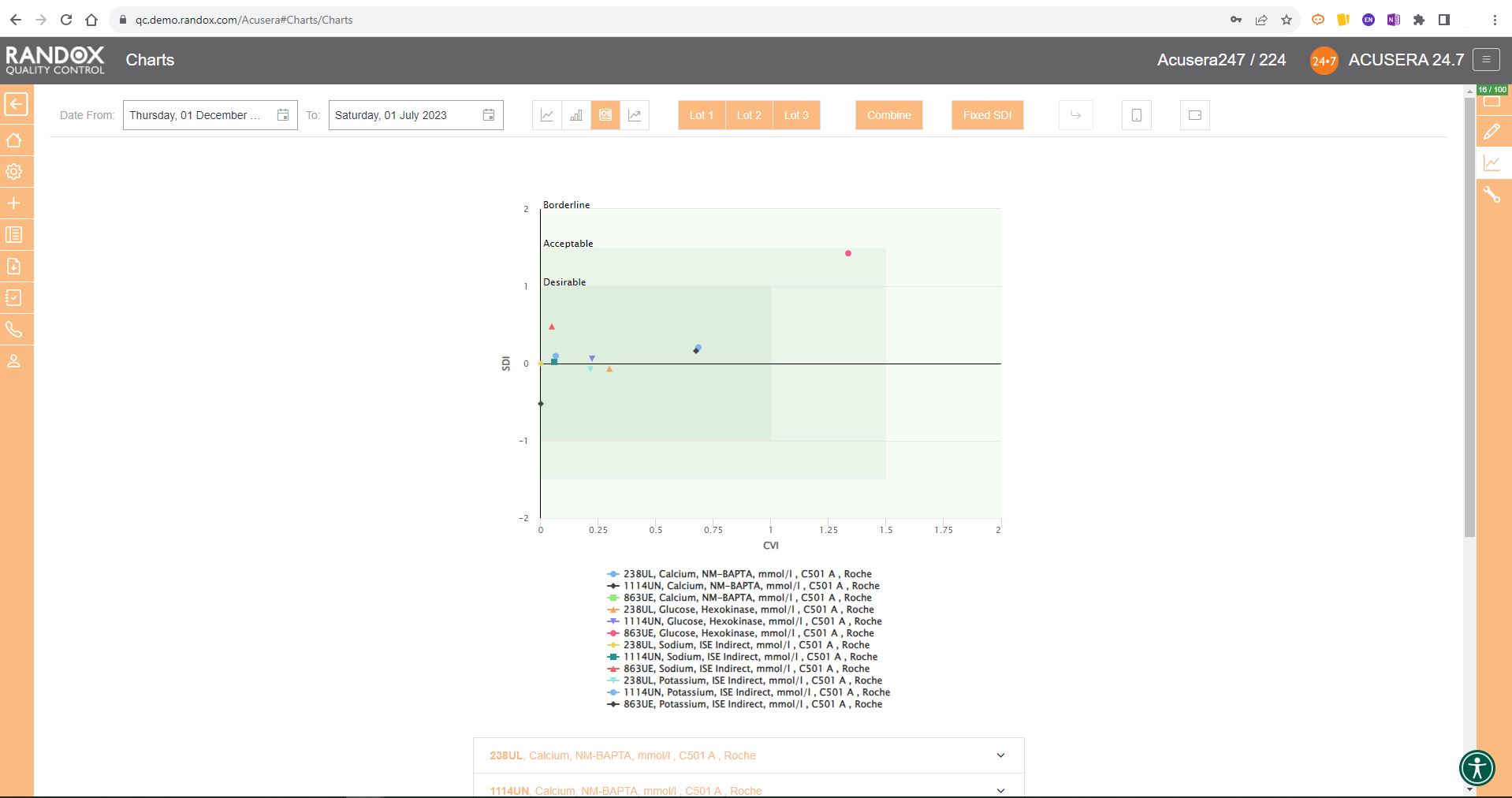
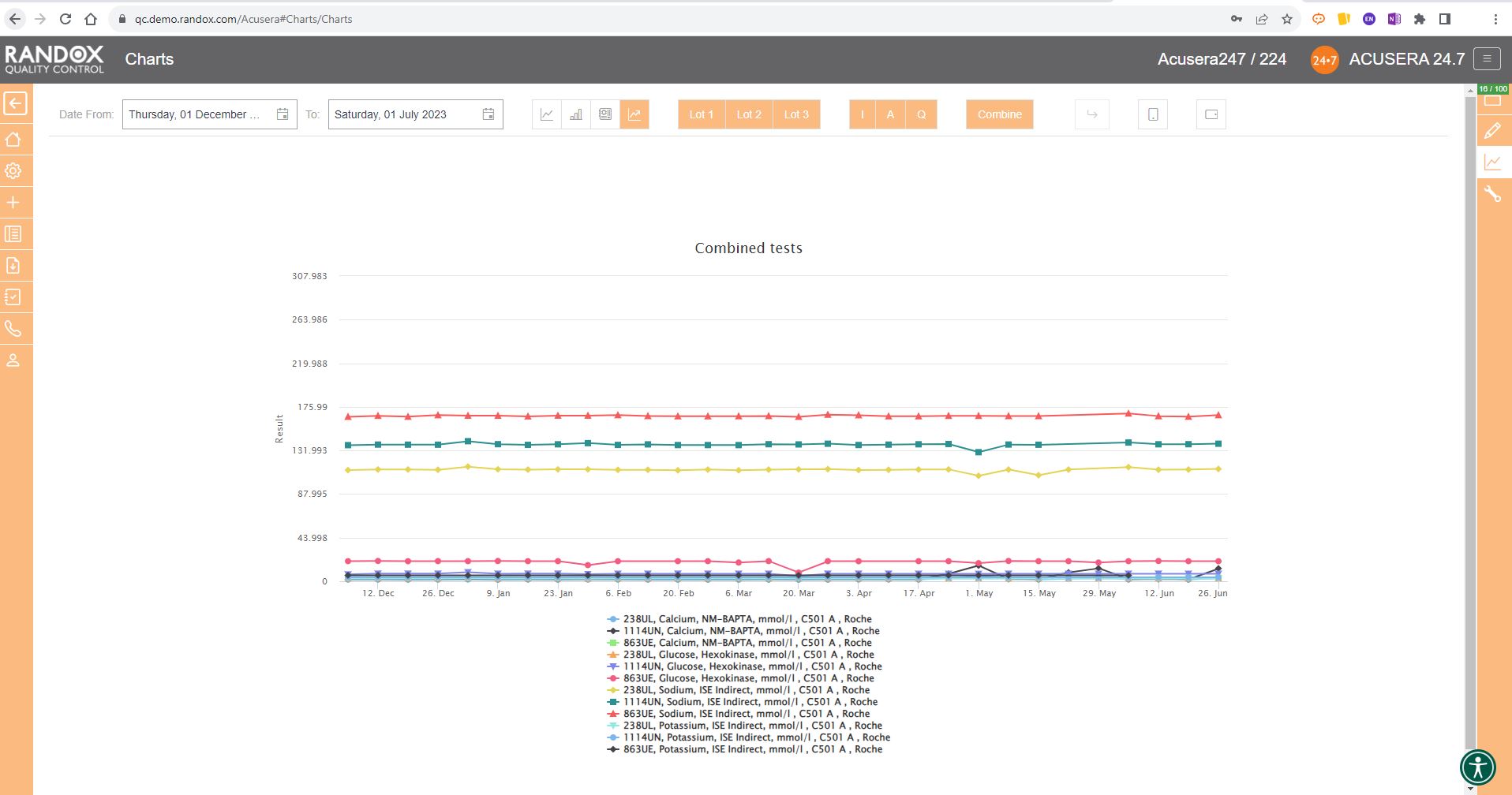
Like the Levey-Jennings charts, you can also combine this information for panels or a selection of multiple lots and analytes. You can see an example below:
Weekly Mean Charts
Weekly Mean Charts are one of the new features in our latest software release.
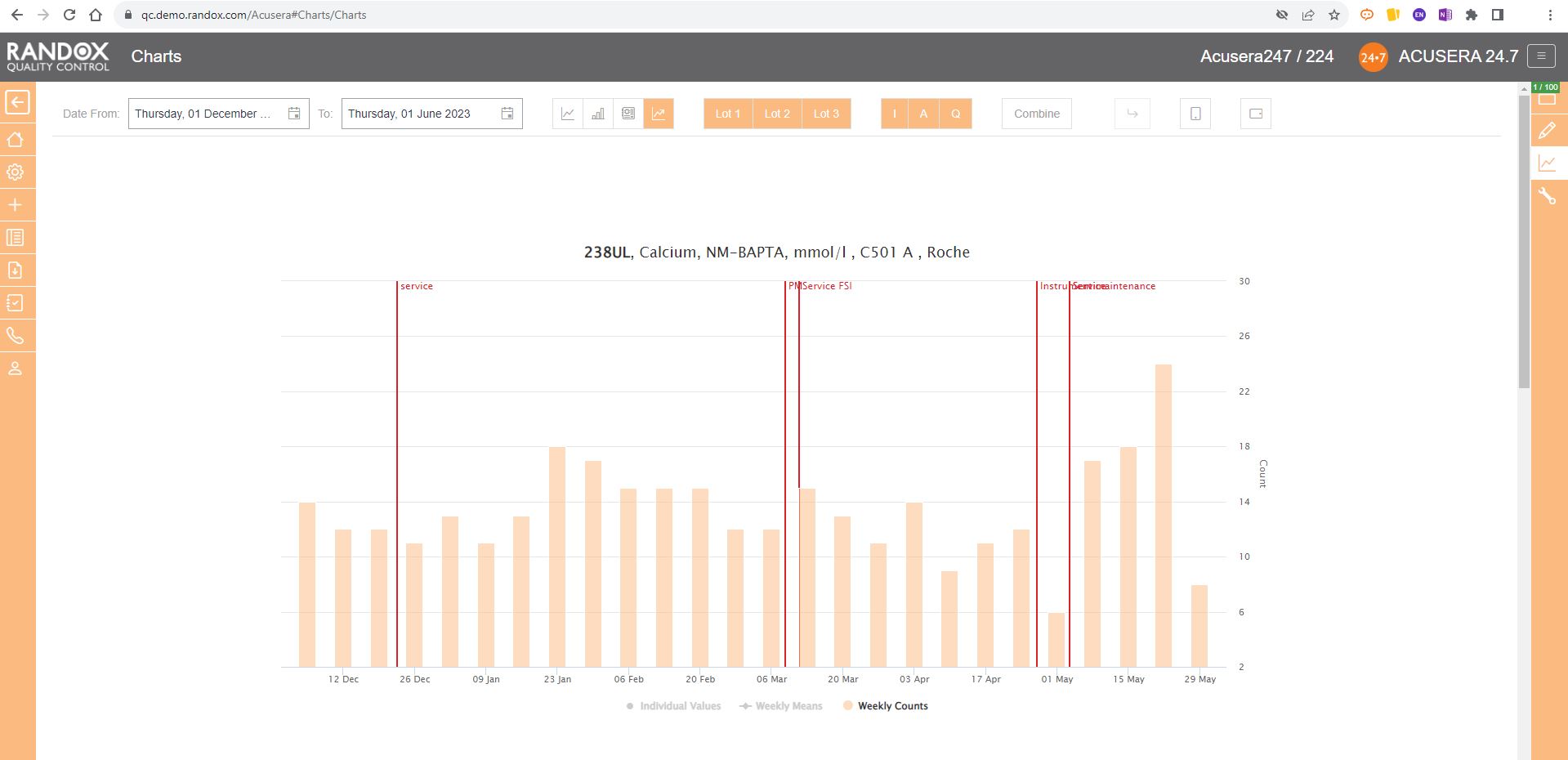
They allow you to view your weekly count of QC results for a specific instrument, assay, or lot.
Below is an example in a bar chart format.
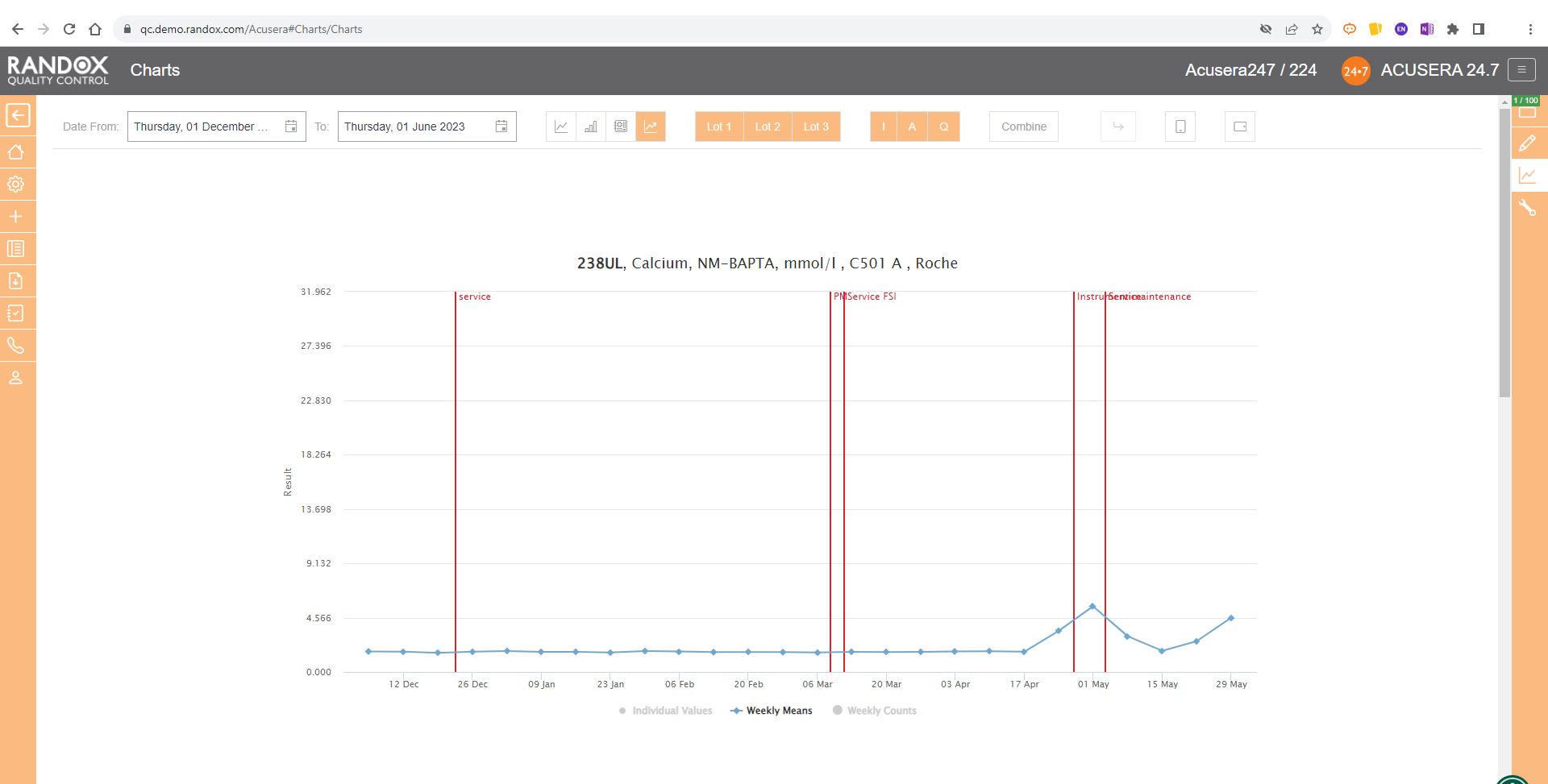
You can also view this data as a line graph, which plots the weekly mean of results from multiple instruments using the same assay and QC lot, allowing a comprehensive overview of your QC data.
Or you can view your weekly means for a range of tests and panels.
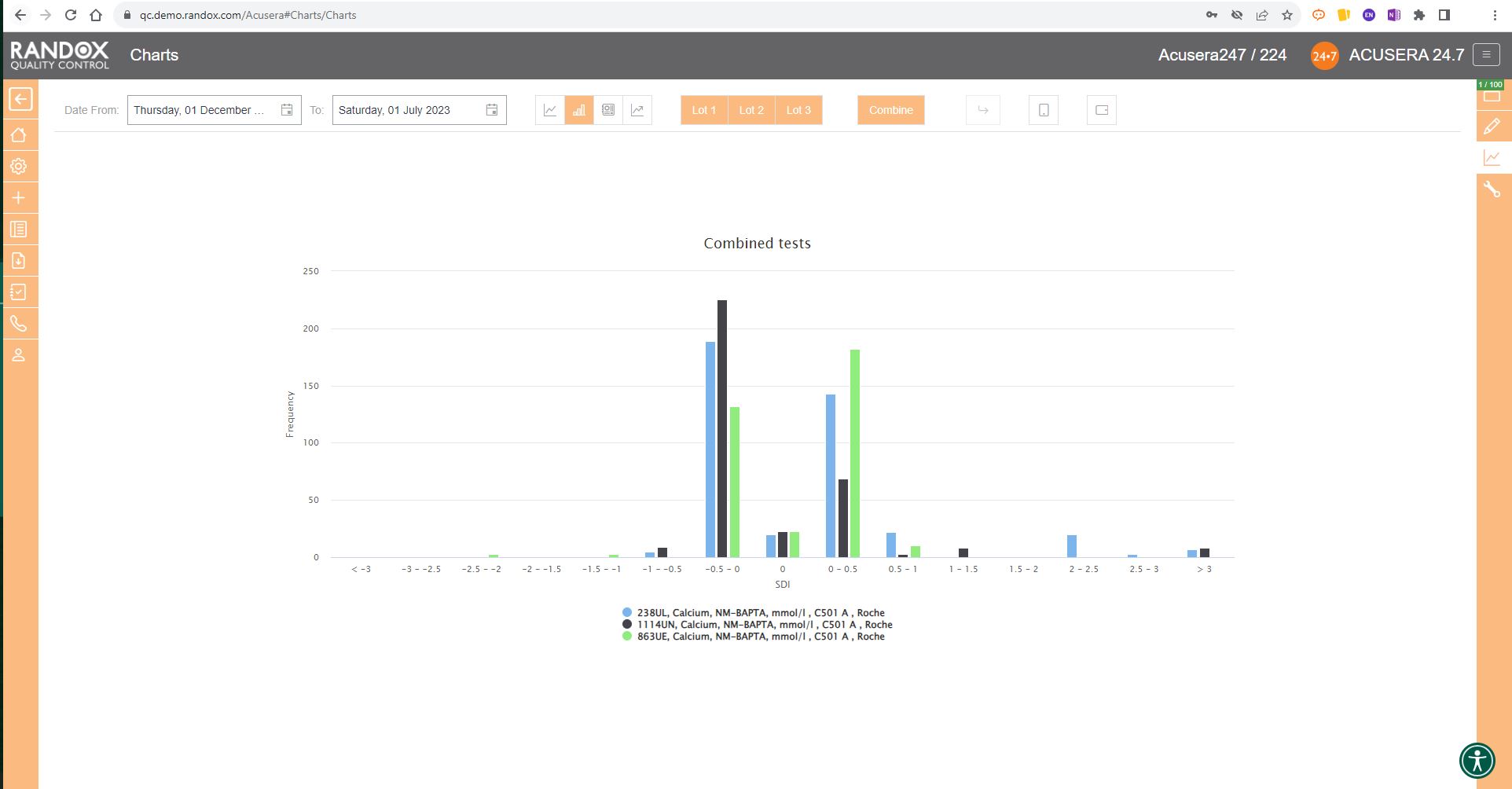
Finally, the SD Histograms allow you to view the distribution of your results, for an overview of performance.
When used with Acusera 24.7’s suite of advanced statistical tools and reports, our charts can help you reduce the time you spend investigating non-conformances.
When the dreaded accreditation assessment approaches, you can relax. While others are scrambling to find documentation, you can rest assured that all the QC data you need is easily accessible.
Assessors love to see Acusera 24.7 load when they enter a laboratory because they understand how much easier QC management is when using our software.
We provide complete onboarding assistance and full training on the software for new customers while delivering prompt and effective customer support for existing users. The Acusera 24.7 and QC operations teams are always eager to help new and existing Acusera 24.7 users with any issues they experience.
To learn more about the features of this ground-breaking software, visit our website here.
Alternatively, feel free to reach out to us at marketing@randox.com for more information or to arrange a demo!
From Fear to Freedom: A QC Data Management Revolution
What if we told you we had a solution to the multitude of monotonous hours spent analysing reams of IQC data and could provide you with an intuitive tool packed with comprehensive and customisable reports, interactive charts, and automated statistical analysis to help improve your QC data management?
Perhaps it sounds too good to be true?
This time, it isn’t.
Uncertainty of Measurement. 6Sigma. QC Multi-rules. These words can strike fear into the hearts of even the most experienced laboratory staff.
With Acusera 24.7, we’ve reached under the bed and forced the monster that is advanced statistical analysis out into the cold.
Acusera 24.7 is a live, cloud-based, interlaboratory QC data management and peer group comparison software.
A mouthful. I know.
But let’s break it down
A live, cloud-based software means you can access your QC data from anywhere, anytime.
Bid farewell to the labyrinth of folders you hunt through when troubleshooting or looking for a specific dataset.
Interlaboratory management describes the momentous task many QC managers face – monitoring the QC performance of multiple laboratories in different locations, ensuring they all maintain the high standards required for accreditation and accurate patient results.
Unlike some big-name subscription services, we encourage you to use our software at different locations to help you monitor all your laboratories and instruments to see how their results stack up against one another.
Acusera 24.7 provides multiple levels of access which are completely customisable. This allows you to grant or restrict access to different parts of the software depending on what is required by your staff. This also allows QC managers to view data from all their sites in one location without needing multiple email chains from each laboratory.
Peer group comparison? Isn’t that what EQA is for?
Well, you would be right.
Yes, EQA does provide a comparison with your peer group, but it doesn’t have exclusive rights.
There are many benefits to comparing your IQC data with your peer group. The real-time comparison data aids with troubleshooting, or you can show off how great you are to your friends and colleagues.
You can select your peer group for an instrument, method and more, providing you with a comprehensive picture of how your laboratory performance compares to your peers using the same lot of control.
There are no submission deadlines. One less thing for you to worry about.
Still think it sounds too good to be true?
Then let’s look at some of the software features and how they can be used to make your daily QC data management easier.
Charts
For many laboratories, review of their QC data is a momentous task involving an abundance of printouts with different data tables and graphs and hastily scribbled notes going back maybe months, if not years.
With Acusera 24.7’s interactive Levey-Jennings charts, you can see the QC data from a specified date range. This helps visualise trends and biases over any period to simplify the troubleshooting and lot validation processes, or, can be used as evidence during accreditation assessments. These charts can be generated for a single analyte or for multiple analytes and QC levels.
You can also add events to the graph to record factors that might impact the performance of your analyser such as preventive maintenance, calibrations or switching QC lots. So, when you come to review the QC data and see a shift in the results, you can see at a glance if there was an explanation for the change in QC results.
What’s more, the points plotted on the chart will appear in orange or red if they trigger your alert or reject protocols respectively. Those that appear as a triangle indicate a comment is attached. Comments can be added to any data point directly on the Levey-Jennings chart, allowing you to record any information relevant to the data, saving you time, not to mention the cost of all those sticky notes.

This complements the Panel feature of the software. Within Acusera 24.7 you can create a panel of tests, for example, a Liver Function Test panel, grouping all the tests together. You can then view all the QC data for this panel at the click of a few buttons. Shown below is the collective data for a clinical chemistry panel.
When you do need the paper copy, all the charts and reports found in Acusera 24.7 can be exported to Excel or PDF for independent analysis or printing, making it easy to bring your data to meetings or for hardcopy filing and audits.
For peer group comparison, you can get a performance summary chart. This chart basically does the analysis for you! You define the date and time range, and the software looks at all the data points within it for you and your peer group, comparing individual data, means, CVs and SDs. Like our other charts, you can combine any number of these for multi-analyte analysis.
Advanced Statistics
Some people love statistics. Others can think of nothing worse.
Either way, there’s a lot of work involved in advanced statistical analysis.
Even if you’re in the love camp, you might find yourself sickened before you’ve finished this metaphorical jar of marmite.
The role of a pathology laboratory is not to run QC and show off their statistical skills, but to provide accurate and appropriate patient results.
As the old saying goes, time is money.
But in your case, time is the difference between a fast or delayed diagnosis for a patient.
This may impact their condition or treatment.
By making use of the suite of statistical options included in Acusera 24.7, including QC Multi-rules, 6Sigma and Uncertainty of Measurement, you can focus on providing the most accurate and efficient testing for patients.
Data Entry
To save even more time, Acusera 24.7 can be integrated with many LIMS or Middleware packages for fully automated data transfer. At a predefined time, your internal software will send your QC data to a shared folder on your network and from there to a Randox Cloud IP address, meaning we don’t go into your IT system and take anything; we won’t cause any information security problems. This data is then taken from the cloud and populated onto 24.7.
All this in less time than it takes you to say, ‘fully automated data transfer.’
You can also import your data through a semi-automated upload procedure. For this, the data is exported from your LIMS or middleware and imported manually to your Acusera 24.7 account using an EDI import file. Simply put, all you have to do is send the file, and the software will populate it onto the system. Alternatively, you can upload the data manually on the simple and intuitive data entry page.
Acusera 24.7, while comprehensive and initially daunting due to its vast array of features, is incredibly easy to use. The Acusera 24.7 and QC operations teams are always eager to help new and existing Acusera 24.7 users with any issues they experience. We provide complete onboarding assistance and full training on the software for new customers while delivering prompt and effective customer support for existing users.
We’ve only begun to cover the range of features available on Acusera 24.7 for QC data management! For more information or to arrange a demo, get in touch with our team at marketing@randox.com. Or, you can take a look at our website here.
Pursuing Perfection: Insights into Global IQC Practices
In a time when medical laboratory personnel are pushed to their limits, internal quality control and quality management are easy to consider a nuisance. However, these processes are vital to ensure accuracy and precision in the potentially life-saving tests performed in these laboratories. Most High-to-middle-income countries have strict regulations governing quality procedures in medical laboratories, but global standardisation in these areas is lacking. Over 70% of clinical decisions are based on laboratory testing but many clinicians are unaware of the accuracy and precision limitations associated with many of these tests. This places the responsibility on laboratory staff to ensure that all results provided to clinical decision-makers are as true as possible. For this, they rely on IQC and a robust quality management system.
To determine the state of the industry, a report by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Task Force on Global Laboratory Quality (TF-GLQ) surveyed over 100 IFCC full and affiliate members, receiving responses from 46 countries1. This survey consisted of a series of multiple-choice questions in relation to quality practices in their respective countries.
Findings by IFCC Task Force on Global Laboratory Quality
90% of respondents indicated that quality standards are in use in their country, despite being mandatory in only 46.7% of those countries.
These responses are encouraging showing that at least some level of predefined QC practice is implemented even in countries that do not legislatively mandate the inclusion of quality standards. This also hints that in those countries where it is not mandatory, it may soon become a requirement to adhere to a specified QC system. Nevertheless, in countries where regulatory measures are currently absent, the rigour of the implemented quality control procedures may not be adequate to ensure the accurate reporting of results.
42.5% of respondents indicated that IQC was not run in all laboratories in their country.
These respondents indicated that IQC is run in 50-99% of laboratories in their country. This less encouraging result shows that minimum IQC practices are not implemented globally. However, due to the multiple-choice nature of this survey, it is difficult to determine how drastic this issue is. Although it does raise the question of how these laboratories verify the precision of their results.
66.7% of respondents indicated that they use assay manufacturer quality control material.
This refers to first party quality control materials which are optimised by the manufacturer for use with a specific assay, instrument or method. These controls are often manufactured from the same material as the calibrator, making them less sensitive to subtle changes in performance, allowing them to mask weaknesses in the assay in question and therefore should be considered less effective options than third-party controls. Additionally, ISO15189:2022 encourage the use of third-party controls and require laboratories seeking accreditation that do not use third party controls to provide a sufficient explanation as to why this is the case.
60% of respondents indicated that not all laboratories in their country had written IQC policies and procedures.
This highlights an important aspect of a quality management system. Without written IQC policies and procedures it is almost impossible to standardise the IQC process and corrective action across laboratory staff, never mind on a national scale. Drafting this documentation can be cumbersome, however, many organisations can be contracted to assist with the drafting and implementation of these procedures for laboratories seeking to gain accreditation.
28.6% of respondents reported that manual interpretation of the IQC data was normal practice.
Manual data interpretation also poses challenges to the standardisation of IQC processes. Written IQC policies and procedures are crucial in implementing standard acceptance criteria for IQC results. Manual data interpretation also implements restrictions on the ability to carry out more advanced statistical analysis of the QC data.
Discussion
The implementation of robust IQC practices is crucial for ensuring the trueness and precision of the results produced by a laboratory. Used correctly, IQC can monitor variability caused by instrumentation and lot changes as well as various other sources of analytical error. ISO15189:2022 provides a thorough framework for designing rigorous IQC policies and procedures, highlighting key areas such as the use of third party QC material, levels of QC material, the frequency at which IQC should be completed, matrix composition, acceptance/rejection criteria and non-conformance procedures. For more information on ISO15189:2022 accreditation, take a look at our educational guide ISO15189:2022 Updates.
The results from this survey conducted by IFCC show a clear disparity between IQC processes around the globe, displaying differences in requirements, recommendations, and legislation. Standardisation of IQC is not without its challenges. However, by striving to achieve the highest possible levels of quality, and following the guidance laid out in ISO15189:2022, laboratories can be confident in the results they provide to clinicians.
Acusera Quality Control
The Acusera range offers unbiased, independent third party quality controls for medical and research laboratories of all shapes and sizes. Our assayed controls are provided with target values for most commercially available analysers, ensuring that your test menu will be covered. With enhanced stability, commutability and consolidation, all our controls are manufactured to provide a clinically relevant challenge to your test method, aiding in ISO15189 accreditation. For more specialist laboratories, our teams are happy to discuss your requirements and help to provide bespoke quality control material, providing an extremely flexible QC range.
Acusera 24.7
Designed for use with the Acusera range of third party controls, the Acusera 24•7 software will help you monitor and interpret your QC data. Access to an impressive range of features, including interactive charts, the automatic calculation of Measurement Uncertainty & Sigma Metrics and live peer group data generated from our extensive database of laboratory participants, ensures Acusera 24•7 is the most comprehensive package available. For laboratories performing manual review of their IQC data, Acusera 24•7 provides a comprehensive yet easy-to-use platform for advanced statistical analysis and monitoring of these data.
For more information on our Acusera range of IQC material, or Acusera 24•7, feel free to reach out to us at marketing@randox.com or alternatively, browse our range of literature at the QC Resource Hub
References
- Wheeler SE, Blasutig IM, Dabla PK, et al. Quality standards and internal quality control practices in medical laboratories: an IFCC global survey of member societies. Clinical Chemistry and Laboratory Medicine (CCLM). 2023;0(0). doi:10.1515/cclm-2023-0492
World Hepatitis Day 2023
Introduction
World Hepatitis Day, observed on July 28th, serves as a crucial reminder of the ongoing battle against hepatitis (HBV), a viral infection that affects millions of people worldwide. In 2019, it was estimated that 296 million people were living with chronic hepatitis B, resulting in over 800,000 fatalities1. In this article, we will delve into the intricate mechanisms behind hepatitis, explore the viral species responsible for its occurrence, discuss methods for diagnosis, and shed light on treatment and management strategies.
Understanding Hepatitis
Hepatitis refers to the inflammation of the liver, often caused by viral infections. Among the primary hepatitis viruses are Hepatitis A, B, C, D, and E, each with distinct modes of transmission and characteristics2.
Mechanisms of Hepatitis Infection
Hepatitis A and E: Hepatitis A and E viruses are primarily transmitted via the faecal-oral route, often through contaminated food or water. Ingestion of these viruses leads to acute infection, and while self-limiting in most cases, they can cause significant morbidity and mortality in certain populations5,6.
Hepatitis B, C, and D: Hepatitis B, C, and D viruses are predominantly spread through blood and bodily fluids. Hepatitis B can also be transmitted from mother to child during childbirth which in endemic areas, HBV infection from mother to child transmission accounted for approximately half of chronic infections. These viruses can cause chronic infections, leading to long-term liver damage, cirrhosis, and an increased risk of hepatocellular carcinoma7,8.
Diagnosis of Hepatitis
Accurate and timely diagnosis of hepatitis is crucial for appropriate management. Diagnostic methods include:
Serology: Serological tests, such as enzyme immunoassays, are employed to detect specific viral antigens or antibodies in blood samples, aiding in the identification of different hepatitis viruses and determining the stage of infection9.
Nucleic Acid Testing: Highly sensitive molecular techniques like polymerase chain reaction (PCR) enable the detection and quantification of viral genetic material, aiding in the diagnosis and monitoring of chronic hepatitis10.
Treatment and Management of Hepatitis
The management of hepatitis depends on several factors, including the virus involved, the stage of infection, the presence of co-infections, and the individual patient’s health status. Treatment strategies encompass:
Antiviral Medications: For hepatitis B and C, antiviral drugs such as interferons and direct-acting antivirals have revolutionized the treatment landscape, offering higher cure rates and improved outcomes11,12.
Supportive Care: Hepatitis patients may require supportive care to alleviate symptoms, maintain proper nutrition, and manage complications. Vaccination against hepatitis A and B is highly recommended for prevention13.
Liver Transplantation: In cases of end-stage liver disease or hepatocellular carcinoma resulting from chronic hepatitis, liver transplantation may be considered a lifesaving option14.
Randox Hepatitis Solutions
Acusera
Acusera provides a range of positive and negative serology controls comprising various infectious diseases including Hepatitis. The table below details the suitable controls, and more information can be found on our website: Serology Quality Controls – Randox Laboratories

RIQAS
The RIQAS HIV/Hepatitis EQA programme is designed to monitor the performance of tests used to detect HIV/Hepatitis antibodies and specific antigens. All samples are conveniently supplied liquid ready-to-use and are suitable for qualitative methods of analysis.
Parameters:
- Anti-HIV-1
- Anti-HCV
- Anti-HTLV-II
- HBsAg
- Anti-HIV-2
- Anti-HBc
- Anti-HTLV-1&2 (combined)
- Anti-HIV-1&2 (combined)
- Anti-HTLV-I
- Anti-CMV
- Anti-HAV IgM
- Anti-HAV (Total)
- Anti-HBc (Total)
- Anti-HBe (Total)
- Anti-HBs (Total)
- P24
For more information, please visit our website at: HIV Hepatitis EQA | RIQAS (randox.com)
Qnostics
Monitoring for the presence of Blood Borne Virus (BBV) nucleic acid is an essential parameter in guiding clinical treatment and patient outcomes. The use of appropriate quality control measures is important in ensuring the appropriate daily performance of the molecular assay used in the laboratory independent of the technology.
Qnostics’ Blood Borne Virus Molecular Controls comprises a range of pathogens which are classically detected directly from the blood including those related to hepatitis. The table below lists the Qnostics products related to hepatitis testing. For more information visit our website: Qnostics | Molecular Infectious Disease Controls – Randox Laboratories

QCMD
QCMD is a world-leading External Quality Assessment (EQA) / Proficiency Testing (PT) scheme, dedicated to improving the quality of molecular diagnostic assays used in the detection of infectious diseases. With an extensive database of over 2000 participants in over 100 countries, QCMD is one of the largest providers of molecular EQA in the field of molecular diagnostics. QCMD programmes related to hepatitis testing are listed below:
- HBV Drug resistance Typing EQA programme.
- HCV Drug resistance Typing EQA programme.
- Hepatitis B Virus DNA EQA Programme
- Hepatitis B Virus Dried Blood Spot EQA Pilot Study
- Hepatitis B virus Genotype EQA Programme
- Hepatitis C Virus Dried Blood Spot EQA Pilot Study
- Hepatitis C Virus RNA EQA Programme
- Hepatitis C virus Genotype EQA Programme
- Hepatitis D Virus EQA Programme
- Hepatitis E virus RNA EQA Programme
For more information on any of these EQA programmes please visit: QCMD – Molecular EQA Scheme | Randox Quality Control
Conclusion
World Hepatitis Day serves as a reminder of the global impact of hepatitis and the urgent need to raise awareness, prevent transmission, and improve the diagnosis and management of this disease. By understanding the mechanisms, bacterial species involved, diagnostic techniques, and treatment approaches, we can work towards a future free from the burden of hepatitis. Let us unite in our efforts to combat this disease and strive for a healthier world.
If you’d like to find out more about hepatitis or the diagnosis and testing of hepatitis, please visit our website. If you’d like more information on how Randox can improve hepatitis testing in your laboratory, please reach out to marketing@randox.com.
References
- World Health Organization. World Health Statistics 2023. World Health Organization; 2023. https://www.who.int/publications/i/item/9789240074323
- World Health Organization. Hepatitis. https://www.who.int/news-room/fact-sheets/detail/hepatitis-a. Published 2017. Accessed June 9, 2023.
- Wan Z, Wang X. Bacterial Hepatitis. In: Encyclopedia of Medical Microbiology. Elsevier; 2020:110-117.
- Russo TA, McFadden DC. Bacterial and fungal infections in patients with cirrhosis. Clin Liver Dis. 2019;14(2):71-74.
- World Health Organization. Hepatitis E. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e. Published 2018. Accessed June 9, 2023.
- Rakesh S, Pekamwar SS. Hepatitis A. In: StatPearls [Internet]. StatPearls Publishing; 2020.
- World Health Organization. Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Published 2021. Accessed June 9, 2023.
- World Health Organization. Hepatitis D. https://www.who.int/news-room/fact-sheets/detail/hepatitis-d. Published 2021. Accessed June 9, 2023.
- Alfaresi MS, Elkoush AA, Khan AS. Serological diagnosis of viral hepatitis. J Clin Transl Hepatol. 2017;5(4):343-359.
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C. J Hepatol. 2017;66(1):153-194.
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370-398.
- Vermehren J, Sarrazin C. New HCV therapies on the horizon. Clin Microbiol Infect. 2011;17(2):122-134.
- World Health Organization. Hepatitis A. https://www.who.int/news-room/fact-sheets/detail/hepatitis-a. Published 2020. Accessed June 9, 2023.
- Kim WR, Terrault NA. Hepatocellular carcinoma and liver transplantation. Clin Liver Dis. 2018;22(2):381-394.
Acusera 24·7 Software Updates v3.3
Randox Quality Control is thrilled to announce the release of our latest software update for Acusera 24·7, which includes a collection of new features to enhance your user experience and create a more effective quality management system for your laboratory. This update shall take place on Tuesday 20th June 2023. Below, you’ll find details of the latest software updates and how these changes can help you improve your daily QC activities.
Events
- Users can now add an event at the assay, instrument or QC levels to allow more accurate monitoring of control events. In addition, this feature adds the capability to record reagent lot changes.
- Users now have the ability to temporarily hide all events from the interactive charts, allowing for a clearer view of QC performance over a selected timeframe.
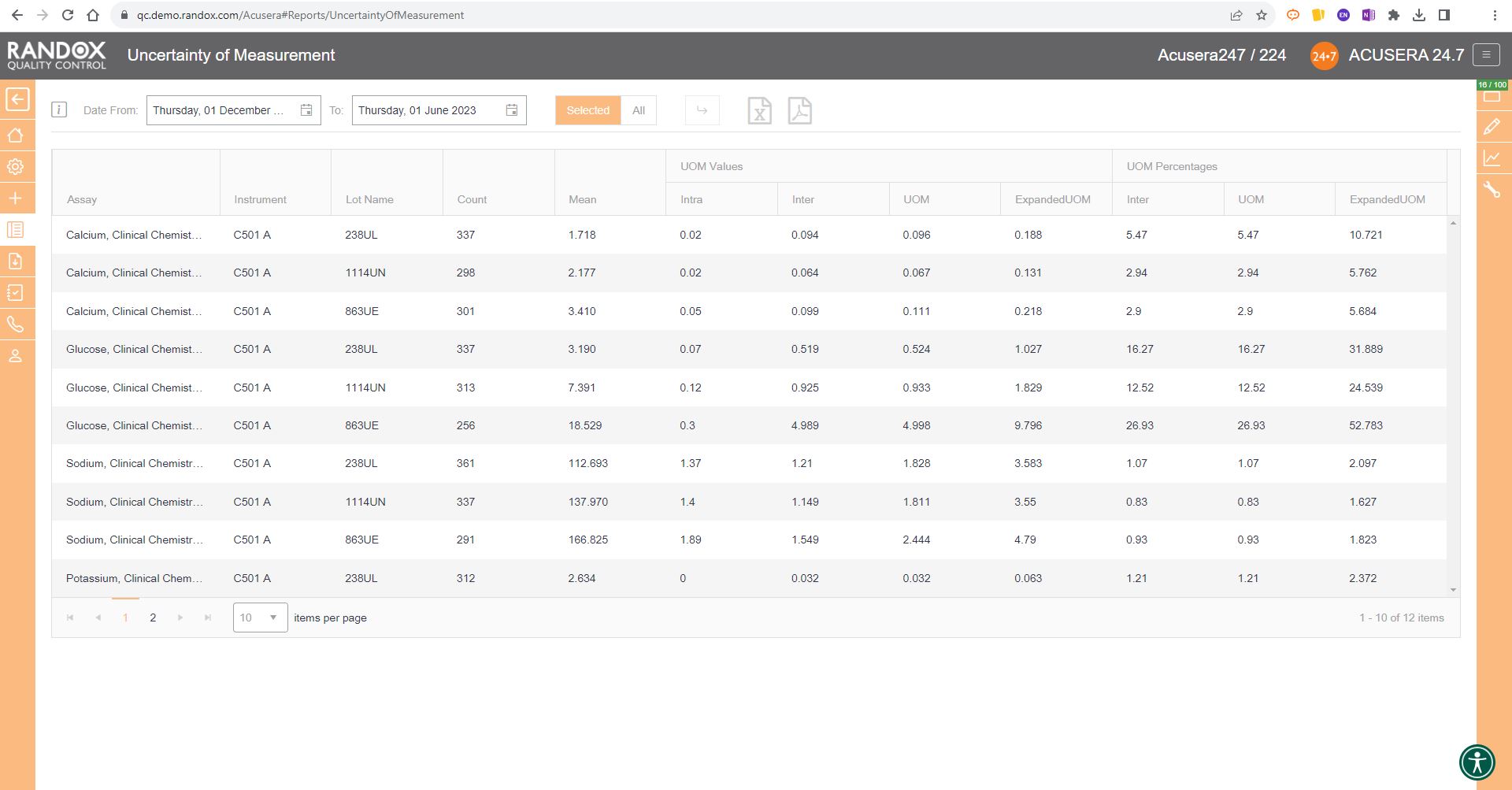
Uncertainty of Measurement
- User can now add the uncertainty of the calibrator value to the uncertainty of measurement report to provide a more accurate assessment of uncertainty.
- Users now have the ability to hide the intraprecision data from the uncertainty of measurement report if no data has been entered for this field.
Charts
- A selection of new interactive charts have been added to this software. These charts focus on the individual results per analyte that each instrument generates over a specified time period.
Individual results
This graph displays a spread of the individual results for a single machine, per analyte generated over a specified time.

Weekly Count
This Bar Chart shows the weekly count of quality control results for a specific instrument, assay and lot.

Instrument Comparison
Users can now view a line graph, which plots the weekly mean of results from multiple instruments using the same assay and QC lot, allowing a comprehensive overview of your QC data.

If you’d like to learn more about these updates, we encourage you to watch our new Acusera 24·7 video guides: Acusera 24.7 Video Guides
This software update will be live from Tuesday 20th June 2023. To make the upgrade process as smooth as possible, we encourage Acusera 24·7 users to clear their browser cache, visit the Acusera 24·7 site, and you will be ready to avail of these new features!
If you would like an additional information on these updates, or anything else relating to Acusera 24·7, don’t hesitate to reach out the marketing@randox.com. Additionally, feel free to visit our QC resource hub where you will find all of our brochures, support tools and a collection of educational material, to aid you in maintaining the highest possible levels of quality.

Enhancing Laboratory Quality Control with Multi-Rule QC: A Comprehensive Guide
Introduction
We are thrilled to announce the release of our latest educational guide, “Understanding Multi-rule QC,” which delves into the world of laboratory quality control. Designed for laboratory professionals, this comprehensive guide aims to empower you with knowledge and strategies to ensure accurate results and uphold patient safety.
Understanding the Significance of Multi-Rule QC
Laboratory quality control is paramount in maintaining the integrity of test results. The guide begins by exploring the various causes of deviations in laboratory testing processes. From instrument malfunctions to environmental factors, we shed light on potential sources of error that can impact result accuracy.
Next, we dive into the core of the guide: Multi-rule QC. This powerful framework encompasses a series of rules that serve as a robust screening tool for identifying outliers, shifts, and trends in data. Through an in-depth exploration of rules such as 1:2s, 1:3s, 2:2s, R4s, 3:1s, 4:1s, 10x, and 7T, we unveil their underlying principles and their significance in maintaining quality control within laboratory settings.
Applying the Multi-Rule QC Approach
The guide equips laboratory professionals with practical insights on applying the Multi-rule QC approach. By examining consecutive data points, analysing trends, and detecting systematic shifts, you gain the ability to proactively address issues before they compromise result accuracy. We highlight the importance of avoiding overreliance on individual rules for result rejection, emphasizing the need to consider additional factors such as clinical relevance and method performance.
Troubleshooting Out-of-Control Events
No laboratory is immune to out-of-control events. That’s why our guide goes beyond rule implementation and delves into effective troubleshooting strategies. We provide guidance on identifying root causes, implementing corrective actions, and re-establishing control in your laboratory environment. By embracing a culture of continuous improvement, you can minimize the impact of deviations and optimize laboratory performance.
Acusera 24.7
Acusera 24.7 is a cloud-based inter-laboratory data management and peer-group reporting software designed to assist in the management of daily QC activities and aid continuous improvement in the laboratory. It includes multi-rule capabilities that can be utilized to monitor your QC data and index it as accepted, rejected, or trigger an alert, depending on the pre-defined multi-rules against which you want to check your data. These features enable the identification of nonconformities and reduce the need for laborious manual statistical analysis while enhancing the accuracy and precision of the laboratory.
Conclusion
In an era where accuracy and patient safety are paramount, the “Multi-rule QC” guide serves as an invaluable resource for laboratory professionals. By mastering the principles and applications of Multi-rule QC, you can enhance the quality control processes within your laboratory, mitigating risks and delivering reliable test results.
To explore the full potential of Multi-rule QC and embark on a journey of laboratory excellence, we invite you to download the guide today. Stay ahead of the curve and ensure the highest standards of quality and patient care in your laboratory!
You can download the Understanding Multi-rule QC Educational Guide below:
If you’d like to find out more about what we can do to help your laboratory or view our range of Internal Quality Controls, don’t hesitate to contact us at marketing@randox.com or feel free to browse the range on our website https://www.randox.com/laboratory-quality-control-acusera/.

Introducing Comprehensive Educational Guides on Updated CLIA Proficiency Testing Regulations
We are thrilled to present two educational guides that delve into the newly updated minimum performance specifications for Proficiency Testing by CLIA (Clinical Laboratory Improvement Amendments). These regulations, set to be implemented by 2024, aim to enhance the accuracy and reliability of test results in clinical laboratories. Here, we introduce these invaluable resources designed to assist laboratories in navigating the evolving landscape of proficiency testing.
1. Proficiency Testing Regulations Related to Analytes and Acceptable Performance – A Final Rule (Microbiology):
Our first guide focuses on the specific regulations and requirements pertaining to microbiology proficiency testing. With a comprehensive exploration of these guidelines, this guide is a useful resource for microbiology labs striving to ensure precision and integrity in their testing procedures. From the required categories of testing to maintaining optimal testing conditions, the guide details the updates that promote adherence to the highest standards of quality and safety.
2. Proficiency Testing Regulations Related to Analytes and Acceptable Performance – A Final Rule (Non-Microbiology):
For non-microbiology laboratories, our second guide delves into the updated proficiency testing regulations concerning various analytes. From chemistry to haematology, molecular diagnostics to immunology, this guide offers a comprehensive overview of the new requirements and minimum performance specifications. By embracing these regulations, medical laboratories can uphold the utmost accuracy and reliability in their test results, ensuring optimal patient care and clinical decision-making.
Elevating Laboratory Practices:
These educational guides are indispensable tools that empower laboratories to navigate the changing landscape of proficiency testing regulations. By staying informed and adopting the updated minimum performance specifications, laboratories can maintain compliance, demonstrate excellence, and ultimately deliver the highest quality of care to their patients.
Accessing the Guides:
We invite you to access these comprehensive educational guides by following the link provided below. They offer a wealth of knowledge and practical insights, serving as essential references for laboratory professionals, quality managers, and anyone involved in clinical diagnostics.
With the implementation of updated CLIA proficiency testing regulations on the horizon, these educational guides come at a crucial time. By embracing the knowledge and guidance they provide, laboratories can navigate the changing landscape with confidence and ensure their adherence to the highest standards of proficiency testing. Together, let’s strive for excellence, precision, and patient-centric care in clinical laboratory practices.
#CLIARegulations #ProficiencyTesting #ClinicalLaboratories #QualityAssurance #PatientCare

The Importance of Third-Party Controls #LABWEEK2021
In the clinical laboratory, Quality Control (QC) refers to the process of detecting analytical errors to ensure both the reliability and accuracy of patient test results. Poor performance can result in misdiagnosis, delayed/inappropriate treatment, increased costs and may even be potentially life threatening for the patient. Third party controls offer a better solution to ensure optimum performance and accuracy.
When the laboratory professional runs the QC material on their instrument, they can compare the obtained result with the expected result. If these values are comparable, then the laboratory professional can be confident that their instrument is reporting accurately. Essentially, QC is a ‘practice run’ to ensure the testing system is working correctly.
Benefits Of Using Third-Party Controls
Third party controls have been designed to deliver an independent, unbiased assessment of performance with any instrument or method helping you gain accreditation. Below are some of the key benefits of third party controls:
- Values assigned using a large number of independent laboratories ensuring statistically valid targets.
- Highly consolidated controls allow for space, time, and ultimately, cost savings.
- Boosted shelf life ensures continuity of supply and reduced costs
- Reduced preparation times by removing the need for multiple instrument controls
Importance Of Third-Party Controls In The Midst Of COVID-19
Quality Control is a hugely important part of laboratory quality. Around 70% of clinical decisions are made based on laboratory results, so it is plain to see how significant Quality Control practices can be in relation to global health care. Having faith in the performance of the Quality Control used within the lab, as well as benefits gained from highly consolidated controls, allow for space, time, and ultimately, cost savings. These benefits would prove highly beneficial for any laboratory operating in the fast-paced environment posed by the pandemic.
How Randox Can Help
Randox Acusera true third party quality controls offer complete test menu consolidation for laboratory Internal Quality Control. Providing accurate and reliable sample material and delivering results you can trust.
Find out more: https://www.randox.com/laboratory-quality-control-acusera/
Want to know more?
Contact us or visit our Acusera page to learn more.











