![Biotin - landing page banner[2] Biotin](https://www.randox.com/wp-content/uploads/2018/07/xBiotin-landing-page-banner2.jpg.pagespeed.ic.33PpDA8fvo.jpg)
Biotin, also known as vitamin B7, is involved with fatty acid metabolism, amino acid degradation, and gluconeogenesis. The recommended daily intake for biotin is roughly 30-70µg, which is extremely low, meaning that biotin deficiency is rare. Recently, there has been a surge in biotin supplementation mainly for beauty reasons, including: stronger nails and healthier skin and hair, resulting in the biotin craze on Instagram. Currently 162K posts are attributed to the biotin hashtag (#biotin) on Instagram. Whilst biotin supplementation is beneficial for numerous health conditions, including: multiple sclerosis (MS), diabetes, elevated cholesterol, and metabolic dysfunction, the increasing use of biotin by patients has created a problem with in vitro diagnostic testing.
With numerous manufacturers using biotin-streptavidin technology to develop in vitro diagnostic tests, combined with the rise in biotin supplementation use, the FDA (food and drug administration) issued an alert regarding the potential for erroneous results triggered by high levels of biotin in patient samples, at the end of 2017. Clinical decisions based on these false results from biotin technology can lead to inaccurate diagnosis and inappropriate treatment prescribed. The FDA confirmed that a patient, who was consuming high levels of biotin, died when a troponin tested was skewed and failed to show that the patient was having a heart attack. Other tests that can produce erroneous results include: cardiac, pregnancy, cancer and iron-deficiency tests.
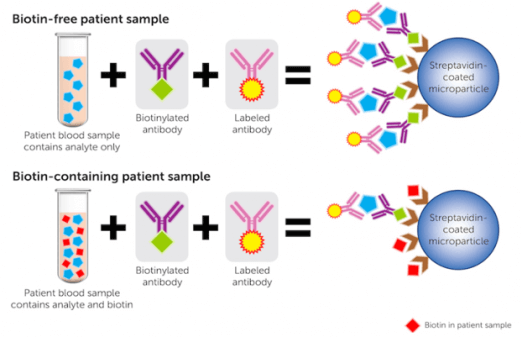
(Halasey, 2018)
The image above highlights that in biotin-free patient samples, the analyte to be tested successfully binds to the biotinylated antibody and the labelled antibody ultimately ensuring accurate measurement. In the patient sample containing high levels of biotin, the biotin inhibits streptavidin’s ability to capture the analyte-antibody complex, generating falsely lowered results.
As 70% of all clinical decisions are based on results from in vitro diagnostic tests, it is vital that laboratories are selecting in vitro diagnostic tests that do not adopt the biotin-streptavidin technology to ensure accurate patient testing.
Randox do not utilise the biotin-streptavidin technology in the development of the Biochip Array Technology (BAT).
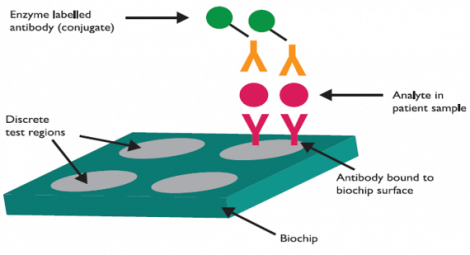
The Randox Biochip facilitates multiplex testing for faster, more comprehensive patient testing. This technology, free from biotin-streptavidin, uses capture antibodies bound to a solid substrate (biochip surface) as opposed to biotinylated antibodies used by other manufacturers. The Biochip also utilises enzyme labelled conjugate to enable chemiluminescent detection of the target in the patient sample.
Biochip test menu

The Biochip Array Technology (BAT) from Randox is capable of simultaneous multi-analyte diagnostic testing within the fields of clinical research and drugs of abuse testing. The technology works through combining a panel of related assays on a single biochip with a single set of reagents, controls and calibrators. An extensive range of Biochip panels are available, each optimised to provide the best performance.
Evidence
The evidence analyser is the world’s first protein Biochip Array Technology system and has truly transformed laboratory diagnostics worldwide. As the first of its kind, the Evidence has introduced higher standards of quality efficiency and reliability to numerous sectors including hospitals and clinical laboratories, forensic and clinical toxicology, pharmaceutical/CRO applications, as well as veterinary laboratories.
Evidence Evolution

The world’s first fully automated random access Biochip testing platform. The Evidence Evolution is set to revolutionise current diagnostic testing. With the capability to process up to 2,640 tests per hour, the Evidence Evolution utilises multiplexing technology, offering advanced test consolidation, patient profiling, a complete system integration, as well as the most comprehensive test menu on the market.
Investigator

The #1 choice for research, clinical, forensic, and veterinary testing. Using the multiplexing technology, the semi-automated benchtop immunoanalyser Evidence Investigator is suitable for medium throughput laboratories. In addition to the current test menu for this analyser, Randox have new tests in development.
Reference:
Halasey. S, 2018, “Inside Track: Biotin Gets a Safety Alert”. Available from URL: http://www.clpmag.com/2018/01/inside-track-biotin-gets-safety-alert/
