Benefits of High-Sensitivity Troponin I (hs-TnI)
Benefits of High-Sensitivity Troponin I (hs-TnI)
Chest pain is a common symptom; 20% to 40% of the population will experience chest pain during their lifetime. There are many causes of chest pain, some of which are benign, while others are potentially life threatening. Importantly, in patients with chest pain caused by an acute coronary syndrome (ACS) or angina, there are effective treatments to improve symptoms and prolong life, emphasising the importance of early diagnosis in patients where chest pain may be of cardiac origin (Skinner et al, 2010). Chest pain is one of the most common reasons for emergency admission to hospital and is a heavy burden on health-care resources. A strategy to identify low-risk patients suitable for immediate discharge would have major benefits (Shah et al., 2015).
RIQAS Liquid Cardiac Programme
Interlaboratory data Management

Ford, C. (2017). Benefits of High Sensitivity Cardiac Troponin I at Admission. Clinical Laboratory Management Association, (July/August 2017), 22-24.
Shah, A., Anand, A., Sandoval, Y., Lee, K., Smith, S., & Adamson, P. et al. (2015). High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. The Lancet, 386(10012), 2481-2488. http://dx.doi.org/10.1016/s0140-6736(15)00391-8
Skinner, J., Smeeth, L., Kendall, J., Adams, P., & Timmis, A. (2010). NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart, 96(12), 974-978. http://dx.doi.org/10.1136/hrt.2009.190066
Meeting ISO 15189:2012 Requirements for Multiple Instruments
Approximately 70% of clinical decisions are based on laboratory test results. Poor laboratory quality can result in unreliable test results ultimately leading to misdiagnosis, inappropriate treatment and may even impact the overall quality of life for the patient. Having multiple instruments can often add to the difficulties faced in labs. The importance of quality medical services is recognised globally with several bodies existing internationally including ISO (International Organisation for Standardisation) who have developed a set of guidelines and quality systems to ensure reliable test results – ISO 15189:2012.
About ISO 15189:2012
ISO 15189:2012 was designed to outline the “requirements for competence and quality that are particular to medical laboratories”. Laboratory competence and quality are critical in patient diagnosis and care to ensure they meet the need of the clinicians & patients. Gaining accreditation to ISO 15189:2012 will assure clinicians employing your services that they will be benefitting from accurate results which have been measured against a consistent standard. You could benefit too from cost savings and enhanced end-user satisfaction.
Gaining Accreditation
ISO 15189:2012 divides the quality requirements of the laboratory into two distinct areas; Internal Quality Control (IQC) and External Quality Assessment (EQA). By combining both you can comprehensively review and monitor the overall performance of your laboratory, including personnel, equipment, and procedures.
A particular requirement of ISO 15189:2012 is:
“Laboratories accredited according to ISO 15189 that have two or more analysers for examinations, should have a defined mechanism for comparison of results across analysers”
How Randox can help labs with multiple instruments?
Randox offers solutions in both IQC and EQA to help your lab meet the ISO 15189 requirements.
RIQAS
Our international EQA scheme is the largest in the world with 45,000 participants in 133 countries.
Multi-Instrument Reports
All RIQAS participants can register up to five separate instruments per programme at no extra cost. Individual reports for each instrument plus a unique multi-instrument report are provided. The multi-instrument report plots the performance of each individual instrument on a single, colour coded Levey-Jennings chart, ensuring instant identification of any differences in instrument performance. Additional sample packs may be ordered as required.
The multi-instrument report includes many of the same statistical features found in the main RIQAS report including; CV%, SDI, RMSDI, %DEV, RM%DEV, Target Score, and RM Target Score.
Acusera 24.7 Live Online
Our stress free QC analysis software is designed to assist in the management of daily QC activities.
Support for multiple instruments
Acusera 24.7 Live Online allows laboratories to conveniently combine multiple instruments as well as analytes and QC lots on a single Levey-Jennings chart, allowing comparative performance assessment and immediate visualisation of any ongoing or emerging trends.
Helping you get accredited
Randox helps you get accredited by offering products from the full spectrum of Quality Control, meaning you never have to look elsewhere. Not all manufactures can offer these features.
To find out more about how we can help you meet ISO 15189 requirements, contact us using the form below.

Mythbusting: ‘Using IQC and EQA From the Same Provider Leads to QC Bias’
Some laboratory professionals believe that using Internal Quality Control (IQC) and External Quality Assurance (EQA, also known as Proficiency Testing) material from the same provider can lead to increased levels of qc bias, or that their test system will not be appropriately challenged. It is important to address these concerns, because some labs may in fact be hindering their own performance by using IQC and EQA material from different sources.
It is important to first understand how IQC and EQA work together to help form a complete Laboratory Quality Management System.
IQC and EQA in Laboratory Quality Management
IQC is a means of monitoring test system precision on a daily basis. IQC effectively evaluates test system performance over time, so that any sudden or gradual shifts in performance can be detected. However, while IQC is an effective performance monitor, it cannot detect more intricate problems like calibration errors or wide acceptable limits provided by some QC manufacturers.
EQA is essential for challenging test system accuracy, and is carried out less frequently than IQC testing. EQA samples are tested ‘blind’ and the results are returned to the scheme organiser. As EQA testing compares an individual lab’s performance to other labs using the same method and instrument, it is a very effective tool for identification of potential issues.
Is there any disadvantage to using IQC and EQA material from the same provider?
The answer to this question depends primarily on the source material of the IQC and EQA. If an IQC provider manufactures their material using artificial additives or components of animal origin, then it will not be suitable to use EQA material from the same provider. Westgard (2011) maintains that using non-commutable IQC or EQA material can lead to results becoming compromised due to matrix effects – something which would not happen using commutable controls.
For example, with Immunoassay testing, non-human components of IQC material interact with antibodies in the reagent in a different way to fully human patient samples – ultimately giving unpredictable shifts, and not adhering to the ISO 15189 requirement to: “use quality control materials that react to the examining system in a manner as close as possible to patient samples”.
However, if the IQC and EQA material is manufactured using a source material which is similar in composition to patient samples (100% human), this commutable control will adequately mimic patient sample performance; meaning labs can use EQA and IQC material from the same provider with confidence that the integrity of their results is maintained.
Conclusion
ISO 15189 also states: “Use of independent third party control materials should be considered…”. In this instance, ‘Independent’ does not mean from a separate provider. It means that the QC material should not be optimized for use on one specific instrument (i.e. not dependent on a single instrument/method type).
No regulatory body states a requirement to use different providers for IQC and EQA material. Indeed, using IQC from one provider and EQA from another provider could increase the risk of labs using non-commutable material.
Labs should use commutable IQC and EQA material for a true assessment of their test system. Randox QC and RIQAS EQA are specifically designed with commutability in mind, giving labs a control which reflects patient sample performance and ensures excellent performance.
How can we help?
To learn how Randox can offer a complete solution for your laboratory, follow the links below or submit a question using the form above.
References
Westgard, S. (2011). Is QC Quality Compromised?. Available: https://www.westgard.com/qc-quality-compromised.htm. Last accessed 31st October 2017.

Got a question?
Acusera Internal Quality Control Analyte List
Quality Control is our passion; we believe in producing high quality material that can help streamline procedures, whilst saving time and money for laboratories of all sizes and budgets. With an extensive product offering comprising third party controls and calibrators, interlaboratory data management, external quality assessment, and calibration verification, you can count on Randox to deliver trustworthy results time and time again. Just ask one of our 60,000 users worldwide.
Our Acusera Internal Quality Control A – Z analyte list highlights how comprehensive our Acusera product portfolio is. Search through the list to see if we have the analyte you require.
Acusera Parameter List
#
5-HIAA
17-OH-progesterone
17β Clostebol
1-25-(OH₂)-Vitamin D
25-OH-Vitamin D
A
α-1-Acid Glycoprotein
α-1-Antitrypsin
α-1-Globulin (Electrophoresis)
α-2-Globulin (Electrophoresis)
α-2-Macroglobulin
α-Fetoprotein (AFP)
α-HBDH
ACE (Angiotensin Converting Enzyme)
Acetaminophen Acid Phosphatase (Non-Prostatic)
Acid Phosphatase (Prostatic)
Acid Phosphatase (Total)
ACTH
Active Vitamin B12 (Holotranscobalamin/HoloTC)
Activated Partical Thromboplastin Time(APTT)
AHD Albumin
Albumin (Electrophoresis)
Aldolase x Aldosterone
Alkaline Phosphatase (ALP)
ALT (GPT)
AMH
Amikacin
Ammonia
AMOZ Amylase
Amylase (Pancreatic)
Androstenedione
Anti-HAV
Anti-HBc
Anti-HBe
Anti-HBs
Anti-HCV
Anti-HIV 1 / 2
Anti-HTLV 1 / 2
Anti-Streptolysin (ASO)
Anti-Thyroglobulin (Anti-TG)
Anti-Thyroperoxidase (Anti-TPO)
Anti-Thrombin III (AT III)
AOZ Apolipoprotein A-I
Apolipoprotein A-II
Apolipoprotein B
Apolipoprotein C-II
Apolipoprotein C-III
Apolipoprotein E
AST (GOT)
β-Globulin (Electrophoresis)
β-2-Microglobulin
BASO-X
BASO-Y
Basophils (BASO)
Basophils % (% BASO)
Bicarbonate
Bile Acids
Bilirubin (Direct)
Bilirubin (Total)
Blood Bone Alkaline Phosphatase (B-ALP)
Borrelia burgdorferi IgG
Borrelia burgdorferi IgM
Brain Natriuretic Peptide (BNP)
C-Peptide
C-Telopeptide
CA 15-3
CA 19-9
CA 72-4
CA 125
Caffeine
Calcitonin
Calcium
Carbamazepine
CEA
Ceftiofur
Ceruloplasmin
Chloramphenicol
Chloride
Cholesterol (HDL)
Cholesterol (LDL)
Cholesterol (Total)
Cholinesterase
CK-MB
CK (Total)
Complement C3
Complement C4
Copper
Cortisol
CRP
Creatinine
Cyclosporine
Cytomegalovirus (CMV) IgG
Cytomegalovirus (CMV) IgM
CYFRA 21
Cystatin C
D-3-Hydroxybutyrate
D-dimer
Deoxypyridinoline
DHEA-Sulphate
DIFF-X
DIFF-Y
Digoxin
Dopamine
E-Selectin (E-SEL)
Eosinophils (EOS)
% Eosinophils (% EOS)
Epidermal Growth Factor (EGF)
Epinephrine
Epstein Barr Virus (EBV) EBNA IgG
Epstein Barr Virus (EBV) IgM
Epstein Barr Virus (EBV) VCA IgG
Estriol
Ethanol
Ethinylestradiol
Ethosuximide
Factor II
Factor V
Factor VII
Factor VIII
Factor IX
Factor X
Factor XI
Factor XII
Ferritin
Fibrinogen
Folate
Fructosamine
FSC-X
FSH
G-6-PDH
γ-Globulin (Electrophoresis)
γGT
Gastrin
Gentamicin
Gestagens (Generic)
GLDH
Glucose
Glutamate
Glutathione Peroxidase (Ransel)
Glutathione Reductase
Glycerol
GM-CSF
Growth Hormone (GH)
Haematocrit (HCT)
Haemoglobin (HGB)
Haemoglobin (Total)
Haemolysis (H)
Haemopioetic Progenitor Cell (HPC)
Haptoglobin
HAV IgM
HbA1c
HBc IgM
HBeAg
HBsAg
hCG
Free β-hCG
Total β-hCG
HDL-3
Helicobacter pylori IgG
Herpes Simplex Virus 1 (HSV-1) IgG
Herpes Simplex Virus 1 (HSV-1) IgM
Herpes Simplex Virus 2 (HSV-2) IgG
Herpes Simplex Virus 2 (HSV-2) IgM
HIV-1 P24Ag
Homocysteine
hsCRP
Icterus (I)
IMIDC
IMIRF
Immature Granulocytes (IG)
% Immature Granulocytes (% IG)
Immature Myeloid Information (IMI)
Immature Platelet Fraction (IPF)
Immunoglobulin A (IgA)
High Sensitivity Immunoglobulin A (hsIgA)
Immunoglobulin E (IgE)
Immunoglobulin G (IgG)
High Sensitivity Immunoglobulin G (hsIgG)
Immunoglobulin M (IgM)
High Sensitivity Immunoglobulin M (hsIgM)
Inhibin A
Insulin
Insulin Like Growth Factor (IGF 1) x
Intercellular Adhesion Molecule-I (ICAM-I)
Interferon-γ (IFN-γ)
Interleukin-Ia (IL-la)
Interleukin-1β (IL-1β)
Interleukin-2 (IL-2)
Interleukin-4 (IL-4)
Interleukin-5 (IL-5)
Interleukin-6 (IL-6)
Interleukin-8 (IL-8)
Interleukin-10 (IL-10)
Interleukin-15 (IL-15)
Iron
Iron (TIBC)
Iron (UIBC)
Kappa Light Chain
Ketones
L-Selectin (L-SEL)
Lactate
Lactate Dehydrogenase (LDH)
Lambda Light Chain
Lambda Light Chain (Free)
LAP
Leptin
Leukocytes
Lipase
Lipemia (L)
Lipoprotein (a)
Lithium
Luteinising Hormone (LH)
Lymphocytes (LYMPH)
% Lymphocytes (% LYMPH)
Magnesium
Matrix Metalloproteinase-9 (MMP-9)
Measles IgG
Mean Corpuscular Haemoglobin (MCH)
Mean Corpuscular Haemoglobin Concentration (MCHC)
Mean Corpuscular Volume (MCV)
Mean Platelet Volume (MPV)
Metanephrine
Methandriol
Methotrexate
Methyltestosterone
Microalbumin
Macrophage Inflammatory Protein-1a(MIP-1a)
Monocytes (MONO)
Monocytes % (% MONO)
Monocyte Chemoattractant Protein-1 (MCP-1)
Mumps IgG
Myoglobin
N-MID Osteocalcin (OC)
N-Telopeptide
NEFA
Neuron-Specific Enolase (NSE)
Neutrophils (NEUT)
Neutrophils % (% NEUT)
Neutrophil Gelatinase-associated Lipocalin (NGAL)
Nitrite
Norepinephrine
Normetanephrine
NT-proBNP
Nucleated Red Blood Cells (NRBC)
Nucleated Red Blood Cells % (% NRBC)
Nucleated Red Blood Cells X (NRBC-X)
Nucleated Red Blood Cells Y (NRBC-Y)
Oestradiol
Osmolality
Osteocalcin
Oxalate
Oxyhaemoglobin
P-Selectin (P-SEL)
Paracetamol
PAPP-A
pCO2
pH
Phenobarbital
Phenobarbitone
Phenytoin
Phosphate (Inorganic)
PIGF
Plasminogen
Plasminogen Activator Inhibitor
Platelet Distribution Width (PDW)
Platelet Large Cell Ratio (P-LCR)
Plateletcrit (PCT)
Platelet (PLT)
Platelet Optical Count (PLT-O)
pO2
Potassium
Prealbumin
Primidone
Procalcitonin
Procollagen Type 1 N-Terminal Propeptide (P1NP)
Progesterone
Prolactin
Protein C
Protein S
Protein (Total)
Prothrombin Time (PT)
Pyridinium Crosslinks
Pyridinoline
PSA (Free)
PSA (Total)
PTH (Parathyroid Hormone)
PTH (Intact)
Quinolones (Generic)
Red Blood Cell Y (RBC-Y) x
Red Blood Cell Distribution Width CV (RDW-CV) x
Red Blood Cell Distribution Width SD (RDW-SD) x
Renin
Resistin
Retinol Binding Protein (RBP)
Rheumatoid Factor (RF)
Rubella IgG
Rubella IgM
Salicylate
Semicarbazine (SEM)
Sex Hormone Binding Globulin (SHBG)
sFlt-1
sLDL
Sodium
Soluble IL-2 Receptor a (sIL-2Ra)
Soluble IL-6 Receptor (sIL-6R)
Soluble Transferrin Receptor (sTfR)
Soluble Tumour Necrosis Factor Receptor 1 (sTNFR I)
Soluble Tumour Necrosis Factor Receptor 11 (sTNFR I1)
Specific Gravity
Streptomycin
Superoxide Dismutase (Ransod)
T Uptake
T3 (Free)
T4 (Free)
T3 (Total)
T4 (Total)
Testosterone
Testosterone (Free)
Tetracyclines (Generic)
Theophylline
Thiamphenicol
Thrombin Time (TT)
Thyroglobulin
Tobramycin
Total Antioxidant Status (TAS)
Toxoplasma gondii IgG
Toxoplasma gondii IgM
Transferrin
Treponema pallidum (Syphilis) IgG
Triglycerides
Trimethoprim
Troponin I
Troponin T
TSH
Tumour Necrosis Factor a (TNFa)
Tylosin
Unconjugated Oestriol
Urea
Uric Acid (Urate)
Urine Osmolality
Urobilinogen
Valproic Acid
Vancomycin
Vanillylmandelic Acid (VMA)
Varicella Zoster Virus (VZV) IgG
Vascular Cell Adhesion Molecule-1 (VCAM-1)
Vascular Endothelial Growth Factor (VEGF)
Vitamin B12
White Blood Cells (WBC)
White Blood Cells Differential (WBC-D)
Zinc
Westgard’s Great Global QC Survey 2017
In a QC survey conducted this year, Sten Westgard reached out to more than 45,000 laboratory professionals to gain a comprehensive view of the world’s Quality Control practices. It was one of the largest surveys that have been conducted and shared publicly.
Read on as we take a summarised look at our favourite bits.
Setting control Limits
Most labs are using their actual performance to set their mean and SD, however, a large percentage of labs still use manufacturer’s ranges, peer group ranges, and other non-individual sources for SD. These ranges can typically be set wider than they would if the ranges were based on their actual mean and SD. This can result in labs releasing incorrect patient results.
Laboratories were asked if they used 2 SD control limits on all tests and it was found that a majority use 2 SD. The strict use of 2 SD can generate a high level of false rejections (9% for two controls and higher for three). This causes a high level of out-of-control events; the use of QC multi-rules is recommended.

The types of Controls used by labs
More than 60% of labs were found to be using manufacturer controls, the drawbacks of which are well known. The latest ISO standards strongly encourage the use of independent / third-party controls. Westgard speculates that this will become a mandatory requirement in the next version of ISO 15189.
Frequency of QC
The first question about frequency asked how often labs ran QC during a run. Respondents reported how often they schedule QC in their labs. Around half only run QC at the beginning of a run with labs running it throughout the day coming in close second. A small proportion of labs reported running QC at both the beginning and the end of a run.
The final, least popular option involves spacing out QC based on test volume, the most scientific method determining how many patient samples can be run between controls without raising the risk of unacceptable results.
The next question asked about the overall frequency of QC. Most labs are meeting the once-a-day minimum standard for CLIA regulations.
“QC frequency remains primarily based on the rotational speed of the earth, not driven by needs of the clinician and patient.” – Sten Westgard
QC Frequency Influences
Regulator and accreditation requirements lead the way in influencing the frequency of QC with manufacturer recommendations, and professional judgement following close behind. Only a quarter of labs use the volume of testing to guide their QC frequency and one in six look to EP23 or IQCP for guidance.
Managing QC
Most labs are using on-board instrument informatics to support their QC charting, followed by LIS charting programs, and peer group software.
Of significance is the number of labs using Excel spreadsheets as their primary QC tool as well as standalone QC programs or even manual graph paper. This could be due to varying technological capabilities where some locations may not have access to, or the funds to afford, informatics.
A combined third of labs are out-of-control every day. In some labs this could be the result of running such a high volume of controls that false rejections are inevitable. However, rationalising in this way can lead to ‘alert fatigue’, where users begin to ignore alert flags and stop troubleshooting.
More than a quarter of labs have an out-of-control flag every few days while another roughly one in six have just one per week. A small number of labs report having few QC flags.
Managing QC Costs
Finally, laboratories were asked about the steps they take to manage QC costs. 60% claimed that they take no steps to manage costs. One in six reduced QC frequency, one in eight switched to cheaper controls, while, worryingly, almost one in ten changed their QC rules or widened limits.
Conclusion
Westgard’s Global QC Survey suggests there exists many inefficient implementations of Quality Control, with plenty of room for improvement. The current state of QC is, like many aspects of healthcare, unsustainable. Labs must adopt better approaches or risk their continuing feasibility, or worse, their patient’s results.
How Randox Can Help
Westgard highlights particular issues with labs mismanaging costs, still using manufacturer controls, and setting control limits – this is where Randox comes in.
Acusera Third Party Controls offer the highest quality solution for any lab – regardless of size or budget. Designed to provide an unbiased, independent assessment of performance, our internal quality controls have not been manufactured in line with, or optimised for use with any particular reagent, method or instrument helping you to easily meet ISO 15189 recommendations. Unrivaled consolidation allows for significant cost savings.
Acusera 24•7 Live Online allows you to automatically apply multi-rules and generate charts to help with setting accurate control limits, helping you get your quality control under control.
Reference: Westgard, S (2017), The 2017 Great Global QC Survey Results
To learn more about how Randox Quality Control can help you improve your QC visit the pages below or fill out the contact form and someone will be in touch.
Randox showcases future-proofing diagnostic technology at MEDICA 2017
Randox Laboratories, the world-leading medical diagnostics manufacturer, is showcasing advancements in laboratory technology at the 2017 MEDICA – World Conference for Medicine conference, being held November 13-16 in Dusseldorf, Germany.
Unveiling its state-of-the-art interactive exhibition stand, Randox will host a series of demonstrations of its innovative analysers including the Evidence Evolution and Rx modena, and a number of exciting advances in laboratory medicine, involving increasing the test menu available to clinicians and improving the connectivity of laboratories across the world to improve overall quality.
“Through our advancements in laboratory innovation, we’re driving an industry-wide evolution” said Randox CEO, Dr Peter FitzGerald.
“Our products are leading the way in innovation and enabling laboratories to transform the way they operate. We will be hosting demonstrations of a wide range of our fully-automated analysers, which are packed with cutting-edge technology and intuitive software. The goal is to provide future-proof diagnostic technology that will create the most efficient and effective laboratories.”
Paving the way is Randox’s patented Biochip Array Technology (BAT). This multi-analyte testing platform is the product of a £250 million research and development project. The ceramic tile measuring 9×9 mm can currently run up to 49 assays simultaneously,100 assays in the near future. This innovation allows the simultaneous quantitative or qualitative detection from a wide range of analytes from a single sample. It is suitable for use in a wide range of laboratories including clinical, research, hospital, veterinary and forensic and clinical toxicology.
To enhance the benefits of BAT, Randox introduced the Evidence Evolution to its stable of immunoassay analyser platforms. The Evidence Evolution is the world’s first fully automated random-access biochip testing platform, capable of delivering 2640 results in one hour, with the first delivered in just 37 minutes.
Joining the Evolution in Hall 3 stand A08 is the RX modena. This highly reliable, precise, fully automated clinical chemistry analyser can run 1200 tests per hour including ISE. When combined with its unrivalled RX series test menu, it offers a winning combination for all large, multi-disciplinary laboratories.
“We develop more new tests than any other diagnostics manufacturer, and one of the products that we’re showcasing at this year’s MEDICA is Adiponectin,” added Susan Hammond, Global Sales Manager at Randox.
“This novel biomarker is a powerful new weapon in the fight against some of the biggest health issues faced throughout the world including diabetes, cancers and cardiovascular disease. As it’s World Diabetes Day on Tuesday 14th November, it’s a great opportunity to draw attention to this array which labs can run as part of their routine testing panel.”
MEDICA attendees will also be among the first to experience the advancements delivered by the latest update for Randox’s Acusera 24.7. This online interlaboratory data management and peer reporting package is now smarter, faster and more powerful than ever before.
Acusera 24.7 is designed to help laboratories efficiently review QC data from all their lab instruments on one central platform, thereby allowing quick and easy identification of QC failures and emerging trends. Unique access to peer group data updated instantly in real-time from our global network of laboratory participants will speed up troubleshooting and help pinpoint the root cause of any QC failures by easily identifying if an issue is isolated or widespread.
The Randox team will be on hand throughout MEDICA 2017 at stand #3A08. To make an appointment in advance, contact them through the Randox MEDICA webpage.
Click here for more information on Randox, or to get in touch, phone the Randox PR Team on 028 9442 2413, or email randoxpr@randox.com
Committed to meeting customers’ needs
At Randox Quality Control, we strive to meet and exceed customer expectations ensuring high quality products and superior customer service are at the top of our priority list.
How can Randox Quality Control help you?
High Quality QC
The Acusera range of true third party controls boasts an impressive range of benefits ultimately designed to help laboratories reduce costs and time while also ensuring an accurate and reliable test system.
The extended shelf life of our controls allows the same lot of control to be used for a period of up to 2 years keeping costly new lot validation studies to a minimum. We may also be able to sequester lots on your behalf.
The availability of commutable controls designed to react to the test system in the same manner as a patient sample and controls targeted at clinical decision levels will not only help you to meet ISO 15189:2012 requirements but will effectively challenge instrument performance.
Click here to find out more about our QC range.
Customer support
The Randox global support network are on hand with expert advice to ensure timely, accurate and helpful resolution of any issues or queries you may have. The added benefit of quick delivery of product orders further highlights how we work with and for our customers to provide the best service available.
Customer Reviews
Don’t believe us? Read a few of the reviews we have received from laboratories around the world;
“I would like to thank the Randox team for the excellent service when helping to reserve and manage our IQC orders, lot numbers and stock.” – Chief Biomedical Scientist, London, 2017.
Request your free QC consultation by contacting us today! Get in touch and we can arrange for your laboratory to have a consultation with one of our Randox QC specialists. Alternatively, if you would like to leave us a review you can do so by emailing acusera@randox.com.
A week dedicated to unsung heroes! – Medical Laboratory Professionals Week 2017
From April 23rd to April 29th we are celebrating Medical Laboratory Professionals Week! This is a week dedicated to raising awareness for those who work in a laboratory & the hard work that goes unnoticed every day in laboratories around the world.
Have you ever wondered what happens between submitting your patient sample and receiving your results? Have you ever wondered who conducts the detailed laboratory testing for your annual check-up such as cholesterol and glucose levels? Or who analyses these results? The answer, a Medical Laboratory Professional (MLP). MLP’s provide up to 70% of the medical laboratory results for physicians and others to make informed decisions about a patient’s diagnosis and aftercare treatment plan. The work that laboratory professionals do each and every day is integral to providing excellent patient care. They perform and interpret billions of laboratory tests every year.
Providing accurate and reliable test results is of the utmost importance for laboratory professionals and also for us at Randox. With a passion for Quality Control, and with more than 30 years’ experience developing Laboratory QC for the in vitro diagnostics market, we believe in producing high quality material designed to streamline procedures, whilst reducing costs in laboratories of all sizes and budgets. These qualities have been reflected in our Acusera true third party quality controls, Acusera 24.7 interlaboratory data management software, Acusera Verify Calibration Verification material and RIQAS, the largest international EQA scheme.
Randox Quality Control would like to take this opportunity to thank all the laboratory professionals around the world and especially our own laboratory staff – you truly are the “Unsung Heroes of Healthcare”.

QC Material Stability – Dig a Little Deeper
QC Material Stability
Stability has a number of different definitions, however, the most relevant to clinical diagnostics, and indeed quality control sera, is the “resistance to chemical change or physical disintegration”. Much like a chain, your quality control system is only as strong as its weakest link, or in this case analyte.
Whilst we appear to be stating the obvious here, this might not be as straightforward as it first appears. The product literature you peruse will help you decide what control best suits your needs, whilst many companies will state their control stability in the literature there are some instances where all may not be as it first appears. It is also important to note that some manufacturers may not make stability claims for some of the analytes listed in their control material. In such instances, you are required to validate these in-house, taking up precious time and resources.
Dig a Little Deeper
Whilst we understand that some analytes do have limitations due to their inherent nature, misleading analyte claims can cost the laboratory both time and money. In a recent survey conducted by Randox, 65.5% of respondents indicated that they felt stability was a ‘Very Important’ QC feature. As such it’s important that you look beyond the sales literature when it comes to control stability. Look out for exceptions in the small print of the control kit inserts. For example, if a control has a stability claim of 7 days at 2-8oC and a routine analyte like Cholesterol has a stability claim of just 2 days at 2-8oC then the true stability of the control is only 2 days. In such instances, there is a lot of potential for waste, as laboratories will be required to prepare a new vial of QC material every 2 days leading to increased costs and time. However, if you dig a little deeper into the controls and always read the small print, you could avoid such issues.
How can Randox Acusera benefit you?
For more than 30 years Randox has been shaping the future of clinical diagnostics with our pioneering high quality, cost effective laboratory solutions. Quality Control is our passion, we believe in producing high-quality material that can help streamline procedures, whilst saving money for laboratories of all sizes and budgets. We pride ourselves in not misleading our customers with false stability claims for our controls. With controls such as our Liquid Cardiac and Specific Proteins Controls, you could benefit from a 30-day open vial stability for all analytes, without exception.
By employing our Randox Acusera quality control materials you could benefit from;
Commutable controls, ensuring a matrix that reacts to the test system in the same manner as a patient sample, enabling an accurate and reliable assessment of instrument performance.
Accurate target values that won’t shift throughout the shelf life of the controls, eliminating the need to spend valuable time and money assigning values in-house.
Consolidation of test menu with controls comprising up to 100 analytes, reducing preparation time and storage space required.
Analytes present at clinically relevant levels ensuring accurate test system performance across the clinical range, maximising laboratory efficiency by eliminating the need to purchase additional high or low-level controls at extra expense.
True third party controls designed to provide an unbiased assessment of performance, our Acusera controls have not been manufactured in line with or optimised for use with any particular reagent, method or instrument.
For more information on any of our products, or to request a consultation from one of our QC Consultants, contact us via acusera@randox.com.
What is Measurement of Uncertainty?
Measurement Uncertainty (MU) relates to the margin of doubt that exists for the result of any measurement, as well as how significant the doubt is. For example, a piece of string may measure 20 cm plus or minus 1 cm, at the 95% confidence level. As a result, this could be written: 20 cm ±1 cm, with a confidence of 95%. Therefore, we are 95% sure that the piece of string is between 19 cm and 21 cm long.
Standards such as ISO 15189 require that the laboratory must determine uncertainty for each test. However, they have not specified how this should be done.
How do we calculate Measurement Uncertainty using QC data?
Employing your QC data to calculate uncertainty makes several assumptions; your test system is under control, the patient samples are treated in the same manner as your controls and gross outliers have been removed. If you choose to use your QC data to calculate this you should ensure that you use a commutable control with a matrix similar to that of a patient sample, with analytes present at clinically relevant levels
To calculate MU, labs must look at the intra-assay precision and inter-assay precision of their test.
Intra-assay precision: Sometimes known as ‘within run’ precision, is where 20 or more replicates of the same sample are run at the same time, under the same conditions (calculated from a single experiment). Intra-assay precision helps to assess systematic uncertainties
Inter-assay precision: Sometimes known as ‘between run’ precision, is where 20 or more replicates are run at different times – e.g. 1 replicate every day for 20 days (can be calculated from routine IQC data). Inter-assay precision can help identify random uncertainties within the test system.
*The Australian Association of Clinical Biochemists (AACB) recommends that at least 6 months’ worth of QC data are used when calculating the inter-assay precision1.
Once the data is collected, you must calculate the standard error of the mean (SEM) of the intra-assay precision (A) and the SD of the inter-assay precision (B) in order to measure the uncertainty (u). Once A and B have been calculated, they need to be squared, added together and the square root of the sum found:
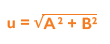
As uncertainty is calculated as SD and 1SD is equal to 68% confidence on a standard Gaussian curve, we can conclude that if we multiply using a coverage factor of 2, we can attain 2SD confidence of 95%. This is known as the Expanded Uncertainty (U):
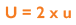
What is the Advantage of Measurement Uncertainty for a lab?
Labs need to carry out MU as it is a requirement of ISO 15189. It states: “The laboratory shall determine measurement uncertainty for each measurement procedure, in the examination phases used to report measured quantity values on patients’ samples. The laboratory shall define the performance requirements for the measurement uncertainty of each measurement procedure and regularly review estimates of measurement uncertainty”.
MU also helps determine whether the difference between two results is negligible due to uncertainty or significant due to a genuine change in condition of the patient; giving labs a greater confidence in reported results.
How can Randox help?
Our new Acusera 24.7 Live Online software provides automatic calculation of MU, saving valuable time and helping labs meet ISO 15189 requirements with ease.
Contact marketing@randox.com to find out how your lab can benefit from Acusera 24.7 Live Online





