Acute Kidney Injury and Antimicrobial Stewardship
Acute Kidney Injury and Antimicrobial Stewardship
An estimated 1 in 5 hospital admissions in the UK is associated with acute kidney injury1, providing a clear illustration of the need for novel, rapid detection methods. Our latest whitepaper looks at this common condition and the links between Acute Kidney Injury and Antimicrobial Stewardship. For more details on the things discussed in this article, you can download the full whitepaper below.
Acute Kidney Injury
Acute Kidney Injury is defined as a sudden loss of kidney function. This causes a disruption in the kidneys’ ability to filter waste out of your blood resulting in an accumulation of waste products as well as other imbalances.
The loss of kidney function is the result of a sudden reduction in glomerular filtration rate (GFR), the process through which waste is extracted from the blood and is often reversible2.
Aetiology of Acute Kidney Injury
The differential pressure existing between the glomerulus and Bowman’s is the driving mechanism for glomerular filtration2. This pressure contrast is influenced by the combined resistances of the afferent (leading to the glomerulus) and efferent (leading away from the glomerulus) vascular pathways in the kidney. Under normal kidney function, these resistances are in equilibrium, facilitating the proper functioning of the GFR. For example, an increase in efferent resistance restricts the blood flow out of the kidney, elevating pressure inside the kidney and reducing GFR, and vice versa2. However, in AKI, the decline in renal blood flow and GFR has a pathological origin. The pathophysiology of AKI can be classified as prerenal, intrinsic renal, or postrenal.
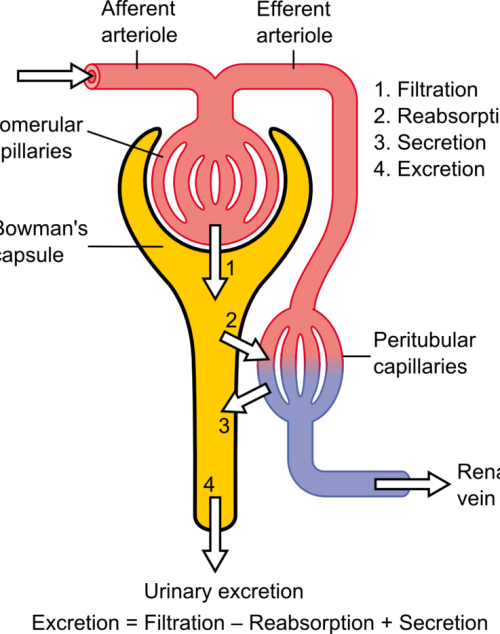
Pre-renal Acute Kidney Injury
Pre-renal AKI is caused by reduced afferent blood flow or, in other words, increased afferent resistance. While tubular and glomerular functions generally remain intact, pre-renal AKI may be caused by systemic hypoperfusion (decreased blood flow) or selective hypoperfusion to the kidney, caused by renal artery stenosis or aortic dissection3.
Intrinsic Renal Acute Kidney Injury
Renal AKI describes the conditions which affect the glomerulus or tubule, for example, acute tubular necrosis and acute interstitial nephritis. This collection of conditions is associated with vasoconstrictor expression in renal afferent pathways2.
Post-renal Acute Kidney Injury
Post-renal AKI usually results from an obstruction in the filtration system. Causes of obstruction include kidney stones, tumours, or blood clots, commonly in the bladder outlet. Obstruction affecting one side might not invariably lead to acute kidney injury, especially when the impediment develops slowly, such as with tumour growth. This is because the unaffected kidney might be able to adjust and make up for the compromised functionality3
Symptoms & Treatment
AKI often manifests with decreased urine output as its primary symptom. However, additional symptoms, when present, can encompass fatigue, nausea, vomiting, or confusion4. To achieve an accurate diagnosis, a comprehensive review of the patient’s medical history and a physical examination are essential to identify the underlying cause of the condition.
The treatment and management of AKI are contingent upon the root cause of the condition. In milder cases, measures are implemented to maintain appropriate levels of fluid, electrolytes, and blood pressure. Nutritional support may also be administered when necessary. In the most severe instances of AKI, dialysis may be warranted to compensate for the diminished kidney function5.
Creatinine serves as a valuable diagnostic tool for evaluating renal conditions, including kidney health, GFR, and muscular dystrophy. However, abnormal serum creatinine (SCr) levels only become evident when a significant portion of the renal mass is compromised. The kidneys possess an impressive capacity to adapt to reduced function, which means that a considerable loss of function or GFR is necessary to influence SCr levels. This poses a challenge when it comes to early detection of AKI6.
Novel biomarkers, KIM-1, NGAL, Clusterin, and Cystatin C, are associated with AKI2 and can be analysed through molecular testing. These new methods can provide a fast and accurate assessment of an individual’s kidney health, at a much earlier stage than SCr quantification2.
Antimicrobial Stewardship
Antimicrobial Stewardship (AMS) programs are specifically crafted to enhance the efficiency of antimicrobial utilization, curtail the emergence of Antimicrobiasl Resistance (AMR), and enhance patient outcomes7. These programs encompass a variety of approaches, such as educational initiatives, training, the establishment of guidelines and protocols, ongoing monitoring and feedback regarding antimicrobial usage, and the management of antimicrobial formularies. Through the promotion of prudent antibiotic utilization, AMS programs contribute to the safeguarding of the efficacy of currently available antimicrobial agents and the deceleration of AMR development7.
Antibiotics and Acute Kidney Injury
Various antibiotics are associated with the progression of AKI due to their nephrotoxicity which can cause severe damage to the kidneys. These antibiotics include polymyxins, aminoglycosides and the commonly used, vancomycin8.

Randox Renal Injury Detection
Using the patented Biochip Technology, the Randox Acute Kidney Injury (AKI) array, available on the Evidence Investigator, simultaneously tests for four novel biomarkers (KIM-1, NGAL, Clusterin, Cystatin C) delivering an early diagnosis and monitoring of treatment efficacy. Multiplex testing better captures reduced renal function, as each biomarker reflects different mechanisms that result in similar injury outputs, allowing for a more accurate picture of the underlying cause of AKI. Along with being able to identify AKI at a much earlier stage, this array provides an accurate and sensitive solution for the diagnosis and monitoring of AKI.
If you’d like some more information on the Randox Acute Kidney Injury Array or would like to add this technology to your laboratory, take a look at our website at https://www.randox.com/acute-kidney-injury/ or get in touch today at marketing@randox.com.

References
- NICE. How common is it? Acute Kidney Injury . Published July 2023. Accessed October 2, 2023. https://cks.nice.org.uk/topics/acute-kidney-injury/background-information/prevalence/
- Adiyanti SS, Loho T. Acute Kidney Injury (AKI) Biomarker.; 2012.
- Manzoor H, Bhatt H. Prerenal Kidney Failure.; 2023.
- NHS. Acute Kidney Injury. NHS. Published 2023. Accessed July 31, 2023. https://www.nhs.uk/conditions/acute-kidney-injury/
- Goyal A, Daneshpajouhnejad P, Hashmi M, Bashir K. Acute Kidney Injury . In: StatPearls [Internet]. StatPearls Publishing ; 2023.
- Rule AD, Lieske JC. The estimated glomerular filtration rate as a test for chronic kidney disease: Problems and solutions. Cleve Clin J Med. 2011;78(3):186-188. doi:10.3949/ccjm.78a.11004
- Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990-1001. doi:10.1016/S1473-3099(17)30325-0
- Clifford KM, Selby AR, Reveles KR, et al. The Risk and Clinical Implications of Antibiotic-Associated Acute Kidney Injury: A Review of the Clinical Data for Agents with Signals from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) Database. Antibiotics. 2022;11(10):1367. doi:10.3390/antibiotics11101367
Chronic Kidney Disease
Early Multiplex Detection of Chronic Kidney Disease from a Single Sample
Chronic Kidney Disease is an abnormal kidney function and/ or structure, present for a minimum period of 3 months.
Typically diagnosed on the basis of serum creatine concentration, however, currently employed urine biomarkers do not reliably detect the early stages of CKD at which intervention could be more effective. Screening for CKD and any subsequent required treatments may then alter the course of early stage CKD and reduce complications and/or the associated health conditions.
Utilising patented Biochip Technology, the Randox Chronic Kidney Disease (CKD) arrays could improve patient risk stratification whilst monitoring the effectiveness of treatment. Diagnosis of CKD at early stages will allow earlier intervention for the treatment of kidney disease, and the prevention of further kidney damage.

Randox Chronic Kidney Disease (CKD) Array I (7-plex)
EGF regulates renal cell proliferation, fibrosis and inflammation and is produced in response to renal injury.
IL-8 endothelial-derived chemokine involved in recruiting neutrophils to sites of injury and stimulating their response.
sTNFR1 is used to identify an increase in inflammatory conditions such as CKD.
FABP1 binds long-chain fatty acids, contributing to reducing oxidative stress in the kidneys.
sTNFR2 is used to identify an increase in inflammatory conditions such as CKD.
D-Dimer is a fibrin degradation product, and an index of both coagulation and fibrinolysis.
MIP-1 alpha plays a roles in inflammatory responses at sites of injury or infection.
Randox Chronic Kidney Disease (CKD) Array II (4-plex)
CRP is an acute phase reactant involved in inflammation.
Cystatin C is well recognised marker of kidney filtration dysfunction and injury.
C3a des Arg is a representative of complement component C3a which produces local inflammatory responses.
NGAL is among the current state-of-the-art in CKD biomarkers.
The Evidence Investigator
Meet the Evidence Investigator
The Randox CKD arrays have both been developed for the Evidence Investigator, a semi-automated benchtop immunoassay analyser.
The CKD array’s would improve patient risk stratification whilst monitoring the effectiveness of treatment. Diagnosis of CKD at early stages will allow earlier intervention for the treatment of kidney disease, and the prevention of further kidney damage. Currently available for RUO.

Want to know more?
Contact us or visit our Investigator Webpage
Jaffe Creatinine Assay
Reagent | Creatinine (Jaffe)
A Marker of GFR Function
Benefits of the Randox Jaffe Creatinine Assay
Excellent precision
The Randox Jaffe creatinine assay displayed a within run precision of < 4.0% CV.
Exceptional correlation
The Randox Jaffe creatinine assay displayed a correlation coefficient of at least r=0.99 when compared to commercially available methods.
Liquid ready-to-use
The Randox Jaffe creatinine assay is available in a liquid ready-to-use format for convenience and ease-of-use.
Calibrator and controls available
Calibrator and controls available offering a complete testing package.
Applications available
Applications available detailing instrument-specific settings for the convenient use of the Randox Jaffe creatinine assay on a variety of clinical chemistry analysers.
Ordering Information
| Cat No | Size | ||||
|---|---|---|---|---|---|
| CR510 | 1 x 200ml (S)(L) | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR3814 | R1 6 x 51ml (L) R2 3 x 28ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR8022 | R1 6 x 68ml (L) R2 6 x 20ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR8316 | R1 4 x 20ml (L) R2 4 x 7ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| (L) Indicates liquid option (S) Indicates standard included in kit |
|||||
Instrument Specific Applications (ISA’s) are available for a wide range of biochemistry analysers. Contact us to enquire about your specific analyser.
More useful information
Creatinine is the end-product of muscle catabolism of creatine. In humans, creatinine production is relatively stable, but mainly depends on muscles mass. Consequently, any physiological changes in muscle mass will cause a variation in the creatinine pool independently of GFR changes. Creatinine is freely filtered by the glomerulus at a constant rate with 10% to 40% secreted by the tubules 1.
According to the National Institutes of health, the overall prevalence of chronic kidney disease (CKD) is approximately 14% 2. Creatinine is the most commonly utilised assay in the assessment of renal function 3. The National Kidney Disease Education Program recommends calculating GFR from SCr. Creatinine measurements are useful in the monitoring of disease progression, with the diagnosis of renal failure when SCr levels are greater than the upper normal interval 4.
Creatinine measurements are useful in the diagnosis and monitoring of diabetic nephropathy, the leading cause of kidney disease in patients commencing renal replacement therapy, affecting 40% of diabetics (type 1 and type 2) 5. The RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) study risk score for end-stage renal disease (ESRD) emphasizes the importance of the identification of elevated SCr, alongside other renal markers, in the prediction of end-stage renal disease (ESRD) development in patients with type 2 diabetes mellitus (T2DM) and nephropathy 6.
Acute kidney injury (AKI) is a common complication in COVID-19 patients 7. The analysis of creatinine in COVID-19 patients on hospital admission and after 2 to 4 days highlighted impaired renal function and is the leading cause of death in these patients 8. The National Institute of Care Excellence (NICE), have set out four guidelines for acute kidney injury in hospitalised suspected or confirmed COVID-19 patients and highlights the importance of creatinine testing 9.
Clinical Chemistry Calibrator
Clinical Chemistry Controls
Clinical Chemistry EQA
References
[2] Gounden V, Bhatt H, Jialal I. Renal Function Tests. Treasure Island: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed 24 July 2020).
[6] Dabla PK. Renal function in diabetic nephropathy. World Journal of Diabetes 2010; 1(2): 48-56
[7] Mahmoudi H, Alikhani MY, Taheri NM, Behzadi A. Assessment of changes in blood urea and creatinine levels in patients with coronavirus disease 2019 (COVID-19). v 2020: https://www.researchsquare.com/article/rs-25164/v1 (accessed 16 July 2020)
[9] National Institute of Care Excellence (NICE). COVID-19 rapid guideline: acute kidney injury in hospital. https://www.nice.org.uk/guidance/ng175/chapter/4-Assessing-for-AKI-in-patients-with-suspected-or-confirmed- COVID-19 (accessed 16 July 2020).
Chronic Kidney Disease
Early Multiplex Detection of Chronic Kidney Disease from a Single Sample
Chronic Kidney Disease is an abnormal kidney function and/ or structure, present for a minimum period of 3 months.
Typically diagnosed on the basis of serum creatine concentration, however, currently employed urine biomarkers do not reliably detect the early stages of CKD at which intervention could be more effective. Screening for CKD and any subsequent required treatments may then alter the course of early stage CKD and reduce complications and/or the associated health conditions.
Utilising patented Biochip Technology, the Randox Chronic Kidney Disease (CKD) arrays could improve patient risk stratification whilst monitoring the effectiveness of treatment. Diagnosis of CKD at early stages will allow earlier intervention for the treatment of kidney disease, and the prevention of further kidney damage.

Randox Chronic Kidney Disease (CKD) Array I (7-plex)
EGF regulates renal cell proliferation, fibrosis and inflammation and is produced in response to renal injury.
IL-8 endothelial-derived chemokine involved in recruiting neutrophils to sites of injury and stimulating their response.
sTNFR1 is used to identify an increase in inflammatory conditions such as CKD.
FABP1 binds long-chain fatty acids, contributing to reducing oxidative stress in the kidneys.
sTNFR2 is used to identify an increase in inflammatory conditions such as CKD.
D-Dimer is a fibrin degradation product, and an index of both coagulation and fibrinolysis.
MIP-1 alpha plays a roles in inflammatory responses at sites of injury or infection.
Randox Chronic Kidney Disease (CKD) Array II (4-plex)
CRP is an acute phase reactant involved in inflammation.
Cystatin C is well recognised marker of kidney filtration dysfunction and injury.
C3a des Arg is a representative of complement component C3a which produces local inflammatory responses.
NGAL is among the current state-of-the-art in CKD biomarkers.
The Evidence Investigator
Meet the Evidence Investigator
The Randox CKD arrays have both been developed for the Evidence Investigator, a semi-automated benchtop immunoassay analyser.
The CKD array’s would improve patient risk stratification whilst monitoring the effectiveness of treatment. Diagnosis of CKD at early stages will allow earlier intervention for the treatment of kidney disease, and the prevention of further kidney damage. Currently available for RUO.

Want to know more?
Contact us or visit our Investigator Webpage
Acute Kidney Injury
Multiplex Detection of Acute Kidney Injury from a Single Sample
Acute kidney injury (AKI) is currently diagnosed using serum creatinine as recommended by the KDIGO guidelines. Serum creatinine however, has poor sensitivity and specificity for AKI lagging behind both renal injury and recovery. There is an immediate need for more sensitive biomarkers to enables earlier identification of AKI, monitor drug toxicity and identify patients at an increased risk of CKD, end-stage renal disease or long-term kidney dialysis.
The National Institute for Health and Care Excellence (NICE) has highlighted that is important that patients are assessed for AKI on admission to hospital or transfer, monitored for AKI throughout their stay and AKI is managed appropriately if it develops.
Utilising patented Biochip Technology, the Randox Acute Kidney Injury (AKI) array simultaneously tests for four novel biomarkers delivering early diagnosis and monitoring of treatment efficacy It may also help you conduct safer and faster clinical trials.
Randox Acute Kidney Injury (AKI) Array (4-plex)
This marker is highly upregulated in kidney tubule cells following nephrotoxic injury severe enough to result in acute renal failure, acute tubular necrosis or acute tubulo-interstitial nephropathy.
Due to its small size and basic pH, Cystatin C is freely filtered by the glomerulus. It is then reabsorbed by tubular epithelial cells and subsequently metabolized. Accumulation of Cystatin C in urine is specific for tubular kidney damage and suggests reduced reabsorption at the proximal tubules as a result of toxicant-induced kidney injury.
Expression of Clusterin is upregulated following a variety of renal injuries and is detectable in urine following acute kidney injury induced by administration of nephrotoxic agents. This occurs before the profound renal transformations that give rise to changes in creatinine and BUN.
KIM-1 is a 30kDa type 1 transmembrane glycoprotein found on actvated CD4+ T cells. It is undetectable in healthy kidney tissue but is expressed at very high levels in proximal tubule epithelial cells in the kidney after toxic injury.
The Evidence Investigator
Meet the Evidence Investigator
The Randox AKI array has been developed for the Evidence Investigator, a semi-automated benchtop immunoassay analyser.
The AKI array would improve patient risk stratification whilst monitoring the effectiveness of treatments & drug toxicity by simultaneously and quantitatively detecting multiple urine biomarkers of kidney damage-related analytes from a single sample.

Want to know more?
Contact us or visit our Investigator Webpage
Enzymatic Creatinine Assay
Reagent | Creatinine (Enzymatic)
A Highly Sensitive And Reproducible Method
Benefits of the Randox Enzymatic Creatine Assay
Superior method
The Randox enzymatic method offers a superior specificity when compared to the traditional Jaffe method.
Excellent precision
The Randox creatinine assay displayed a within run precision of < 2.18% CV.
Exceptional correlation
The Randox enzymatic creatinine assay displayed a correlation coefficient of at least r=0.99 when compared to commercially available methods.
Limited interferences
The Randox enzymatic creatinine assay suffers minimal interferences from Bilirubin, Haemoglobin, Intralipid® and Triglycerides, for truly accurate results and ensures suitability with paediatric samples.
Calibrator and controls available
Calibrator and controls available offering a complete testing package.
Applications available
Applications available detailing instrument-specific settings for the convenient use of the Randox enzymatic creatinine assay on a variety of clinical chemistry analysers.
Ordering Information
| Cat No | Size | ||||
|---|---|---|---|---|---|
| CR2336 | R1 4 x 50ml (S) R2 4 x 10ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR2337 | R1 4 x 100ml (S) R2 4 x 20ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR4037 | R1 4 x 50ml (L) R2 4 x 19.5ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR8122 | R1 4 x 65ml (L) R2 4 x 32.3ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR8317 | R1 4 x 20ml (L) R2 4 x 9.5ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| (L) Indicates liquid option (S) Indicates standard included in kit |
|||||
Instrument Specific Applications (ISA’s) are available for a wide range of biochemistry analysers. Contact us to enquire about your specific analyser.
More Information
The Laboratory Working Group of the National Kidney Disease Education Program (NKDEP) released guidelines for the improvement of glomerular filtration rate (GFR) estimation as well as the measurement of serum creatinine (SCr). The recommendation included the recalibration and standardisation of SCr methods to be traceable to the isotope dilution-mass spectrometry (IDMS) reference method. Two IDMS traceable creatinine methods are commercially available: enzymatic assays and compensated Jaffe assays 1.
Of the two enzymatic assays available, the Randox enzymatic creatinine assay converts creatinine to ammonia (NH3) and I-Methylhydantoin. Ammonia then reacts with α-oxoglutarate in the presence of GLDH with oxidation of the co-enzyme NADPH. The decrease of NADPH is proportional to the creatinine concentration and is measured at 340nm 1, 2.
The Randox enzymatic creatinine assay exhibits high sensitivity and reproducibility with the added advantage of liquid ready-to-use reagents with good stability. The enzymatic method represents an improvement for use in the accurate and reliable determination of creatinine.
Creatinine is the end-product of muscle catabolism of creatine. In humans, creatinine production is relatively stable, but mainly depends on muscles mass. Consequently, any physiological changes in muscle mass will cause a variation in the creatinine pool independently of GFR changes. Creatinine is freely filtered by the glomerulus at a constant rate with 10% to 40% secreted by the tubules 1.
According to the National Institutes of health, the overall prevalence of chronic kidney disease (CKD) is approximately 14% 3. Creatinine is the most commonly utilised assay in the assessment of renal function 4. The National Kidney Disease Education Program recommends calculating GFR from SCr. Creatinine measurements are useful in the monitoring of disease progression, with the diagnosis of renal failure when SCr levels are greater than the upper normal interval 5.
Creatinine measurements are useful in the diagnosis and monitoring of diabetic nephropathy, the leading cause of kidney disease in patients commencing renal replacement therapy, affecting 40% of diabetics (type 1 and type 2) 6. The RENAAL risk score for end-stage renal disease (ESRD) emphasizes the importance of the identification of elevated SCr, alongside other renal markers, in the prediction of end-stage renal disease (ESRD) development in patients with type 2 diabetes mellitus (T2DM) and nephropathy 7.
Acute kidney injury (AKI) is a common complication in COVID-19 patients 8. The analysis of creatinine in COVID-19 patients on hospital admission and after 2 to 4 days highlighted impaired renal function and is the leading cause of death in these patients 9. The National Institute of Care Excellence (NICE), have set out four guidelines for acute kidney injury in hospitalised suspected or confirmed COVID-19 patients and highlights the importance of creatinine testing 10.
Related Products
Clinical Chemistry Calibrator
Clinical Chemistry Controls
Clinical Chemistry EQA
References
[2] Randox Laboratories. Creatinine (Enzymatic UV) CR2336. 2020
[3] Gounden V, Bhatt H, Jialal I. Renal Function Tests. Treasure Island: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed 24 July 2020).
[7] Dabla PK. Renal function in diabetic nephropathy. World Journal of Diabetes 2010; 1(2): 48-56.
[8] Mahmoudi H, Alikhani MY, Taheri NM, Behzadi A. Assessment of changes in blood urea and creatinine levels in patients with coronavirus disease 2019 (COVID-19). v 2020: https://www.researchsquare.com/article/rs-25164/v1 (accessed 16 July 2020)
[10] National Institute of Care Excellence (NICE). COVID-19 rapid guideline: acute kidney injury in hospital. https://www.nice.org.uk/guidance/ng175/chapter/4-Assessing-for-AKI-in-patients-with-suspected-or-confirmed- COVID-19 (accessed 16 July 2020).
Early Detection of Acute Kidney Injury in COVID-19 patients
15 July 2020
The Importance of Early Detection of Acute Kidney Injury in COVID-19 patients
Randox are proud to provide an early detection assay, capable of detecting Acute Kidney Injury in COVID-19 positive patients. AKI is an innovative diagnostic tool with the ability to identify four early and highly sensitive markers of kidney injury.
The National Institute for Health and Care Excellence has highlighted that is important that COVID-19 patients are assessed for AKI on admission to hospital or transfer, monitored for AKI throughout their stay and that AKI is managed appropriately if it develops. (NICE, 2020)
The novel test, which includes biomarkers recommended by the U.S. Food and Drug Administration and the European Medicines Agency detects KIM-I, NGAL, Cystatin C, and Clusterin.
Kidney failure associated with COVID-19 is emerging as a common side effect with further studies underway. Early detection to prevent further renal damage, is vital for an individual’s long-term health, wellbeing and overall survival.
The biomarkers on the Randox AKI Biochip have been identified as more sensitive than traditional testing methods, which, based on urine output and levels of serum creatinine, are grossly insensitive and not specific for the accurate diagnosis and monitoring of AKI.
The Randox AKI assay provides results in 2.5 hours. The new testing panel also facilitates increased lab efficiency and reduced sample prep from the laboratory technician. Using just one urine sample, Randox’s patented Biochip Technology tests for all four AKI biomarkers simultaneously, resulting in time and cost saving benefits, which drive towards an increase in clinical performance.
For further information on our Acute Kidney Injury Array please visit the Randox Biosciences website.
For any other enquiries please email info@randoxbiosciences.com
RESEARCH
BIOPHARMA
CLINICAL LAB
BIOREAGENTS
Cystatin C Assay
Reagent | Cystatin C
An Indispensable Marker of Renal Impairment
Benefits of the Randox Cystatin C Assay

Exceptional Correlation
A correlation coefficient of r=1.00 was displayed when the Randox methodology was compared against commercially available methods.

Excellent Precision
The Randox Cystatin C assay displayed a within run precision of < 4.2%.

Wide Measuring Range
The Randox Cystatin C assay has a measuring range 0.4 – 10mg/l for the comfortable detection of clinically important results.

Dedicated Cystatin C Calibrator and Controls
Dedicated Cystatin C Calibrator and Controls available offering a complete testing package.

Applications Available
Applications available detailing instrument-specific settings for the convenient use of the Randox cystatin C assay on a variety of clinical chemistry analysers.
Ordering Information
| Cat No | Size | Analyser | Easy Read | Easy Fit | |
|---|---|---|---|---|---|
| CYS4004 | R1 2 x 17.6ml (L) R2 2 x 6.1ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| (L) Indicates liquid option | |||||
Instrument Specific Applications (ISA’s) are available for a wide range of biochemistry analysers. Contact us to enquire about your specific analyser.
Diagnostic Uses
Serum creatinine (SCr) is the most commonly utilised screening test for renal impairment; however, SCr can be affected by age, dietary protein intake, ethnicity, gender, and lean muscle mass. Consequently, the sensitivity of SCr for the early detection of kidney disease is poor and not suitable for the renal assessment in the elderly 1.
The biggest drawback of SCr is that up to 50% of renal function can be lost before significant SCr levels become detectable as SCr is insensitive to small changes in GFR. Consequently, treatment is not provided at the appropriate time which can be fatal, and so an earlier and more sensitive biomarker for renal function is imperative 2.
Cystatin C (CysC) is a low-molecular-weight (13.3kDa) non-glycosylated protein belonging to the cystatin protease inhibitor family 2, 3. Formed at a constant rate by all nucleated cells, CysC is freely filtered by the glomerular membrane in the kidneys, reabsorbed and fully catabolised by the proximal renal tubule and is not returned to the bloodstream, and so is the ideal marker of glomerular filtration rate (GFR) 3, 4.
Serum CysC levels are inversely correlated with GFR 3. The main advantage of CysC as a marker of renal function is in the creatinine ‘blind’ area, the elderly and in paediatrics 5. It has been reported that CysC has important associations with mortality across the GFR range, including those who are grouped as ‘preclinical kidney disease’ (GFR between 60 and 90mL/min per 1.73m2). Moreover, CysC has been identified as a stronger predictor of adverse cardiovascular outcomes compared to SCr. Combining SCr, CysC and urine albumin to SCr ratio improves risk stratification for kidney disease progression and mortality 6.
Acute kidney injury (AKI) presents with elevated levels of CysC in those with severe COVID-19 in comparison to those with mild COVID-19. CysC can be utilised to determine the extent of kidney damage as well as distinguishing those with severe and mild COVID-19 7.
Useful Links
Cystatin C Calibrator
Cystatin C Control
A-Z Randox Reagents
Reagents Brochures
References
Publications
H-FABP for Acute Kidney Injury Testing Revealed by Randox
A new testing application for the biomarker Heart-Type Fatty Acid-Binding Protein (H-FABP) has been announced by global diagnostics company Randox Laboratories.
Whilst H-FABP is most commonly recognized as an early biomarker of myocardial infarction, the assay’s clinical utility in cardiac surgery associated acute kidney injury (CSA-AKI) is notable. Studies have shown that patients who developed AKI following cardiac surgery had elevated levels of H-FABP both pre-and postoperatively compared to the patients who did not.
Susan Hammond, Randox Product Specialist, explained the new application for H-FABP;
“Cardiac surgery-associated acute kidney injury (CSA-AKI) is a well-recognized postoperative complication of cardiac surgery and is the second most common cause of AKI in the intensive care unit (ICU) – occurring in up to 30% of patients.
“Several AKI studies exist focusing on the measurement of H-FABP levels before, during and after cardiac surgery, one of which found that the post-operative H-FABP levels in patients who experienced any AKI increased 8-fold. It was also noted that the levels of those with severe AKI increased 13-fold and that 10.8% of patients who died from subsequent AKI all had elevated pre-operative levels of H-FABP.
“The Randox H-FABP assay is therefore an independent marker of AKI following cardiac surgery, and can furthermore be used as a CSA-AKI risk assessment assay even in advance of the procedure.”
It has been identified that certain patient groups are more susceptible to CSA-AKI and vulnerability can depend on age, sex, pre-existing cardiac dysfunction, pre-existing chronic kidney disease (CKD), previous cardiac surgery or comorbidity.
Susan Hammond added;
“The ability to include biomarkers that aid in the risk assessment and treatment plan management of a patient is significant. Utilizing H-FABP alongside traditional biomarkers to assess CSI-AKI risk allows the clinician to gain stronger clinical insight in how to improve patient outcomes.”
Key Benefits of the Randox H-FABP assay
A niche product from Randox meaning that Randox are one of the only manufacturers to offer the H-FABP assay in an automated biochemistry format
CE marked for diagnostic use
Automated assay offering a more convenient and time efficient method for H-FABP measurements compared to traditional testing
Exceptional correlation of r=0.97 when compared against other commercially available methods
Applications available detailing instrument-specific settings for the convenient use of the Randox H-FABP assay on a wide range of clinical chemistry analysers
Liquid ready-to-use format for convenience and ease-of-use
Latex enhanced immunoturbidimetric method delivering high performance compared to traditional ELISA testing
Rapid results within fourteen minutes, depending on the analyser.
Wide measuring range of 0.747 – 120ng/ml for the early detection of clinically important results
Dedicated H-FABP controls and calibrator available offering a complete testing package
Kidney Testing on the Randox Biosciences Evidence Series
March is National Kidney Month, a full month dedicated to raising awareness about kidney disease.
There are two kinds of kidney disease; Chronic Kidney Disease (CKD) and Acute Kidney Injury (AKI).
Chronic Kidney Disease (CKD) affects 3 million people in the UK1. Chronic Kidney is defined as a condition that causes damage and stress on the kidneys, therefore decreasing the ability to keep your body healthy.
The kidneys play a vital role of removing any waste and extra water from the blood to form urine. The kidneys also make hormones that help control your blood pressure, make red blood cells, and keep your bones strong and healthy.2
It is crucial to look after your kidneys. If kidney disease gets worse, the excess waste can build to high levels in your blood, resulting in complications such as high blood pressure, anemia, weak bones, poor nutritional health and nerve damage, as well as increasing the risk of developing heart and blood vessel disease.
Chronic Kidney Disease if often caused by diabetes or having a high blood pressure, which can both be prevented by early detection and treatment. Without treatment the kidney disease will worsen resulting in kidney failure which requires dialysis or a kidney transplant to maintain life.
Chronic Kidney Disease I:
- Fatty Acid Binding Protein I – FABPI
- Soluble Tumour Necrosis Factor Receptor I – sTNFR I
- Soluble Tumour Necrosis Factor Receptor II – sTNFR II
- Macrophage Inflammatory Protein Iα – MIP-Iα
- Interleukin-8 – IL-8
- Epidermal Growth Factor – EGF
- D-Dimer
Chronic Kidney Disease II:
- Complement C3a Des Arginine – C3a des Arg
- C-Reactive Protein – CRP
- Neutrophil Gelatinase Associated Lipocalin – NGAL
- Cystatin C
Acute Kidney Injury (AKI) is when your kidneys stop working properly. This is caused by reduced blood flow to the kidneys, which often happens as a complication of another serious disease.3 AKI affects one in five people admitted to hospital as an emergency, and is considered deadlier than a heart attack 2.
AKI can be reversible if found and treated quickly. Therefore, it is important, if someone has signs of having AKI, to get it treated promptly. Abnormal levels of salt and chemicals can build up in our bodies which causes organs to fail, resulting in the need for dialysis, or can even cause death. 3
- Osteopontin – OPN
- Serum creatinine – Creatinine
- Serum cystatin-C – Cystatin-C
- Kidney injury Molecule-I – KIM-I
- Urinary neutrophil gelatinase associated lipocalin – NGAL
The Evidence Series of Immunoassay analysers contains four revolutionary Biochip Array Technology platforms including the Evidence, Evolution, MultiSTAT and the Investigator.
Randox’s renal panel is available on our Evidence Investigator Immunoassay Analyser, which is a multiplex testing platform allowing for the simultaneous quantitative or qualitative detection of a wide range of analytes from a single sample. It provides a unique platform for assessment of biological samples in a rapid, accurate and easy-to-use format.
For more information on any of the Evidence Series analysers, please visit https://www.randoxbiosciences.com/ or contact us at info@randoxbiosciences.com
- https://www.kidneycareuk.org/news-and-campaigns/facts-and-stats/
- https://www.niddk.nih.gov/health-information/kidney-disease/kidneys-how-they-work
- https://www.nhs.uk/conditions/acute-kidney-injury/





