Acute Kidney Injury and Antimicrobial Stewardship
Acute Kidney Injury and Antimicrobial Stewardship
An estimated 1 in 5 hospital admissions in the UK is associated with acute kidney injury1, providing a clear illustration of the need for novel, rapid detection methods. Our latest whitepaper looks at this common condition and the links between Acute Kidney Injury and Antimicrobial Stewardship. For more details on the things discussed in this article, you can download the full whitepaper below.
Acute Kidney Injury
Acute Kidney Injury is defined as a sudden loss of kidney function. This causes a disruption in the kidneys’ ability to filter waste out of your blood resulting in an accumulation of waste products as well as other imbalances.
The loss of kidney function is the result of a sudden reduction in glomerular filtration rate (GFR), the process through which waste is extracted from the blood and is often reversible2.
Aetiology of Acute Kidney Injury
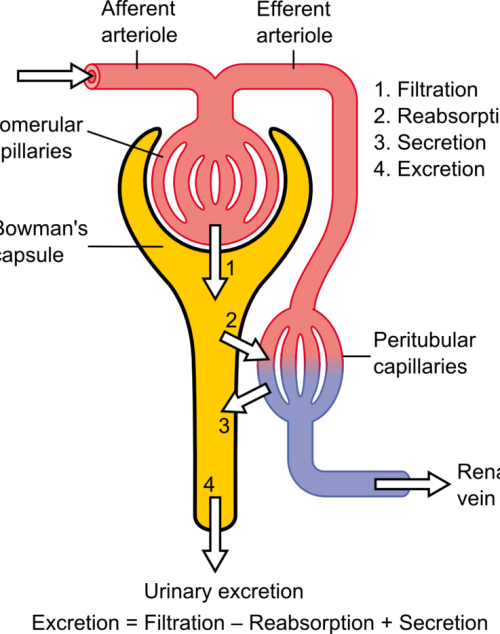
The differential pressure existing between the glomerulus and Bowman’s is the driving mechanism for glomerular filtration2. This pressure contrast is influenced by the combined resistances of the afferent (leading to the glomerulus) and efferent (leading away from the glomerulus) vascular pathways in the kidney. Under normal kidney function, these resistances are in equilibrium, facilitating the proper functioning of the GFR. For example, an increase in efferent resistance restricts the blood flow out of the kidney, elevating pressure inside the kidney and reducing GFR, and vice versa2. However, in AKI, the decline in renal blood flow and GFR has a pathological origin. The pathophysiology of AKI can be classified as prerenal, intrinsic renal, or postrenal.
Pre-renal Acute Kidney Injury
Pre-renal AKI is caused by reduced afferent blood flow or, in other words, increased afferent resistance. While tubular and glomerular functions generally remain intact, pre-renal AKI may be caused by systemic hypoperfusion (decreased blood flow) or selective hypoperfusion to the kidney, caused by renal artery stenosis or aortic dissection3.
Intrinsic Renal Acute Kidney Injury
Renal AKI describes the conditions which affect the glomerulus or tubule, for example, acute tubular necrosis and acute interstitial nephritis. This collection of conditions is associated with vasoconstrictor expression in renal afferent pathways2.
Post-renal Acute Kidney Injury
Post-renal AKI usually results from an obstruction in the filtration system. Causes of obstruction include kidney stones, tumours, or blood clots, commonly in the bladder outlet. Obstruction affecting one side might not invariably lead to acute kidney injury, especially when the impediment develops slowly, such as with tumour growth. This is because the unaffected kidney might be able to adjust and make up for the compromised functionality3
Symptoms & Treatment
AKI often manifests with decreased urine output as its primary symptom. However, additional symptoms, when present, can encompass fatigue, nausea, vomiting, or confusion4. To achieve an accurate diagnosis, a comprehensive review of the patient’s medical history and a physical examination are essential to identify the underlying cause of the condition.
The treatment and management of AKI are contingent upon the root cause of the condition. In milder cases, measures are implemented to maintain appropriate levels of fluid, electrolytes, and blood pressure. Nutritional support may also be administered when necessary. In the most severe instances of AKI, dialysis may be warranted to compensate for the diminished kidney function5.
Creatinine serves as a valuable diagnostic tool for evaluating renal conditions, including kidney health, GFR, and muscular dystrophy. However, abnormal serum creatinine (SCr) levels only become evident when a significant portion of the renal mass is compromised. The kidneys possess an impressive capacity to adapt to reduced function, which means that a considerable loss of function or GFR is necessary to influence SCr levels. This poses a challenge when it comes to early detection of AKI6.
Novel biomarkers, KIM-1, NGAL, Clusterin, and Cystatin C, are associated with AKI2 and can be analysed through molecular testing. These new methods can provide a fast and accurate assessment of an individual’s kidney health, at a much earlier stage than SCr quantification2.
Antimicrobial Stewardship
Antimicrobial Stewardship (AMS) programs are specifically crafted to enhance the efficiency of antimicrobial utilization, curtail the emergence of Antimicrobiasl Resistance (AMR), and enhance patient outcomes7. These programs encompass a variety of approaches, such as educational initiatives, training, the establishment of guidelines and protocols, ongoing monitoring and feedback regarding antimicrobial usage, and the management of antimicrobial formularies. Through the promotion of prudent antibiotic utilization, AMS programs contribute to the safeguarding of the efficacy of currently available antimicrobial agents and the deceleration of AMR development7.
Antibiotics and Acute Kidney Injury
Various antibiotics are associated with the progression of AKI due to their nephrotoxicity which can cause severe damage to the kidneys. These antibiotics include polymyxins, aminoglycosides and the commonly used, vancomycin8.

Randox Renal Injury Detection
Using the patented Biochip Technology, the Randox Acute Kidney Injury (AKI) array, available on the Evidence Investigator, simultaneously tests for four novel biomarkers (KIM-1, NGAL, Clusterin, Cystatin C) delivering an early diagnosis and monitoring of treatment efficacy. Multiplex testing better captures reduced renal function, as each biomarker reflects different mechanisms that result in similar injury outputs, allowing for a more accurate picture of the underlying cause of AKI. Along with being able to identify AKI at a much earlier stage, this array provides an accurate and sensitive solution for the diagnosis and monitoring of AKI.
If you’d like some more information on the Randox Acute Kidney Injury Array or would like to add this technology to your laboratory, take a look at our website at https://www.randox.com/acute-kidney-injury/ or get in touch today at marketing@randox.com.

References
- NICE. How common is it? Acute Kidney Injury . Published July 2023. Accessed October 2, 2023. https://cks.nice.org.uk/topics/acute-kidney-injury/background-information/prevalence/
- Adiyanti SS, Loho T. Acute Kidney Injury (AKI) Biomarker.; 2012.
- Manzoor H, Bhatt H. Prerenal Kidney Failure.; 2023.
- NHS. Acute Kidney Injury. NHS. Published 2023. Accessed July 31, 2023. https://www.nhs.uk/conditions/acute-kidney-injury/
- Goyal A, Daneshpajouhnejad P, Hashmi M, Bashir K. Acute Kidney Injury . In: StatPearls [Internet]. StatPearls Publishing ; 2023.
- Rule AD, Lieske JC. The estimated glomerular filtration rate as a test for chronic kidney disease: Problems and solutions. Cleve Clin J Med. 2011;78(3):186-188. doi:10.3949/ccjm.78a.11004
- Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990-1001. doi:10.1016/S1473-3099(17)30325-0
- Clifford KM, Selby AR, Reveles KR, et al. The Risk and Clinical Implications of Antibiotic-Associated Acute Kidney Injury: A Review of the Clinical Data for Agents with Signals from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) Database. Antibiotics. 2022;11(10):1367. doi:10.3390/antibiotics11101367
Diagnosing UTI Complications in Mothers and Newborns
Diagnosing UTI Complications in Mothers and Newborns
Urinary tract infections (UTIs) are one of the most common bacterial infections that occur in humans. Over 50% of women become infected with a UTI at least once in their lives, with up to 10% of women suffering from yearly infections5. Recurrence rates are high in UTIs, almost 50% of women who contract a UTI experience reinfection or relapse within one year of the initial infection5. Men are four times less likely to contract a UTI due to a longer urethra seen in men when compared with women.
Infections occur in the urinary organs and structures which can be categorized by the site of infection: cystitis (bladder), pyelonephritis (kidney) and bacteriuria (urine)5. So-called, uncomplicated UTIs are sited only in the bladder, however, UTIs are highly likely to cause secondary infections, commonly in the kidneys. Pyelonephritis has been shown to result in renal scarring and in some cases, subsequent renal failure2. There are various species of bacteria responsible for UTIs, which have different mechanisms of infection and virulence. However, most species have surface adhesins which function like hooks, attaching the bacteria to the urothelial mucosal surface, and colonizing the bladder. From here, the bacteria can ascend the ureters, reaching the kidney and causing secondary infections2.
Under normal conditions, the innate immune system actions an inflammatory response to the infection site. However, some species of bacteria that cause UTI can inhibit or delay the immune response resulting in secondary infections in the ureters and kidneys where the risk of severe renal defects is considerable, and the bacteria have direct access to the bloodstream2.
Common symptoms of UTI include:
- Frequent urination
- Painful urination
- Incomplete voiding of the bladder
- Pelvic, back, and/or abdominal pain
- Haematuria
- Lethargy
- Nausea and/or vomiting
- Fever
Antibiotic therapies are effective and aim to facilitate the immune response and inhibit the spread of the infection to the kidneys and upper urinary tract. Although these treatments are usually effective, antimicrobial resistance (AMR) has become a global crisis encompassing all medical disciplines3. This resistance to antibiotics can occur through several mechanisms such as dysregulation of protein expression, structural modifications, and mutations to name a few11.
Bacteria are capable of some level of intrinsic resistance, or insensitivity, to antibiotics through the production of various enzymes designed to degrade the drug or inhibit its mechanism11. Mutations found in the genome of bacterial species are often responsible for the resistance they display. These mutations commonly alter the bacterial binding sites used by antibiotics, therefore inhibiting their action. Some bacteria produce enzymes, which alter the chemical structure of the antibiotic, again, inhibiting them from binding to the antibiotic. Other examples include horizontal gene transfer and biofilm formation10.
One study reported in 2019, that AMR was the twelfth leading cause of death when compared with a susceptible infection counterfactual9. The same study went on to show that AMR had the highest mortality rate in low to middle-income countries providing evidence that AMR is an even bigger problem in the most impoverished parts of the world. New techniques such as CRISPR-Cas9 and antibiotic re-sensitization methods are at the forefront of the fight against AMR, however, the scale of the problem warrants taking all possible action to elevate the risk posed by AMR8.
UTI During Pregnancy
UTIs are a common occurrence in pregnancy with one hospital reporting over 15% of pregnant women being diagnosed with some form of UTI4. Diagnosis can usually be confirmed by a bacterial growth of over 105 counts/ml in urine4, 12, 13. Many hormonal and anatomical changes occur in a woman’s body during pregnancy that create favorable conditions for UTI. Firstly, the glomerular filtration rate is altered, causing an increase in glucose concentration and pH of the urine3. The urethral dilation, smooth muscle relaxation, enlarged mechanical compression of the uterus, and increased plasma volume result in lower urinary concentration and increased bladder size leading to urinary tract reflux and urine stagnation. These conditions are favorable for the proliferation of bacterial infections1.
Diagnosis of UTIs in pregnant women can be complicated. For example, the increased frequency of urination experienced could also be caused by additional pressure placed on the woman’s bladder by the baby, or the abdominal pain indicative of a UTI could be interpreted as Braxton Hicks contractions and vice versa3. There are several established risk factors associated with UTI in pregnancy including advanced maternal age, diabetes, sickle cell anemia, history of UTI, urinary tract abnormalities, and various immunodeficiencies3. Other reports claim that UTI in pregnancy is more common in women with hypothyroidism and women who are carrying their first child4.
Bacterial Species Responsible for UTI
There are a multitude of bacterial species responsible for UTIs, the most common is Escherichia coli (E. coli), followed by group B streptococcus (GBS), enterococcus, and Klebsiella pneumonia. Escherichia coli infections are categorized as either enteric or extraintestinal (ExPEC). Of the latter, there are two main culprits: neonatal meningitis E. coli (NMEC) and uropathogenic E. coli (UPEC)2. These infections can exist in the gut and spread, colonizing other parts of the host such as the blood or central nervous system, causing other potentially severe infections. Of these strains, UPEC is responsible for around 80% of both symptomatic and asymptomatic UTIs. UPEC strains have been associated with acute renal damage and are thought to encourage bacterial growth and persistence by inhibiting or delaying the innate immune response2.
Maternal and Perinatal UTI Complications
UTI complications in mothers and children have long been debated. However, there is sufficient evidence to support several prognostic claims. Preterm delivery is a major complication associated with UTI and has been well studied. Preterm neonates face a high risk of fatality with up to 1 million babies dying every year due to premature labor6. Those that survive are at risk of developing one or more of the following health defects1:
- Lung problems
- Diabetes
- Heart Disease
- Hearing loss
- Visual impairment
- Learning disabilities
- Behavioral problems
- Cerebral palsy
The risk of preterm birth in women who suffered from a single UTI was increased when compared to women who had no infection during their pregnancy but recurrent UTIs did not increase the risk3. Risk of low birth weight has been shown to increase by 50% in women who suffered symptomatic UTIs compared to those who remained uninfected throughout their pregnancy; this risk can be mitigated through antibiotic therapy. The same treatments did not show any significant ameliorative effects on preterm birth4. Women who contract a UTI during pregnancy are also at a higher risk of various conditions such as preeclampsia, postpartum endometritis, sepsis1, hypertensive disorders, anemia and amnionitis4.
Asymptomatic UTIs, also known as asymptomatic bacteriuria (ASB), are not known to cause as drastic primary effects on pregnancy as seen with symptomatic infections. Despite this, ASB can spread and colonize in the kidneys. At this point, pyelonephritis is likely to occur, increasing the risk of severe renal scarring4 and advanced risk of preterm birth3. In these cases, it is common to treat the patient with antibiotics to reduce the risk of a secondary, symptomatic infection. While these treatments are effective at limiting the progression of the infection, overuse of antibiotics is a primary factor contributing to antimicrobial resistance4.
Screening and Treating UTI Complications
Women who are not pregnant and show no risk factors can be tested for UTI through a simple urine dipstick. The presence of leukocyte and absence of nitrite can be considered a positive UTI diagnosis. However, where complications are likely, a urine culture is required. Cultures can be carried out on blood or MacConkey agar and require preservation of the sample in boric acid, or in a refrigerator, for 24 hours prior to testing. This culture can then be isolated and used to identify the strain of bacteria causing the infection7.
Species identification is imperative in maternal UTIs. Different species have different levels of sensitivity to the various antibiotics available. E. coli, for example, shows 93% sensitivity to Nitrofurantoin but is only 86% sensitive to Fosfomycin. Selection of the correct treatment can ameliorate symptoms rapidly and reduce the possible complications for both mother and baby4. Many species of bacteria known to be responsible for UTIs have displayed resistance to antibiotics. Group B streptococcus has been shown to be 42% resistant to clindamycin4. The selection of antibiotics available to clinicians treating maternal UTI are already limited as many antibiotics have been associated with increased risk of miscarriage and birth defects independent of UTI1.
With the patient in mind, Randox provides clinicians with both laboratory and near patient testing solutions. Bringing to the market, to help eliminate distress and improve testing turnaround times, the Randox Urinary Tract Infection Array. It has the ability to detect 30 bacterial, fungal, and associated antibiotic resistance markers from a single urine sample in under four hours. This multiplex diagnostic tool can help detect specific bacterial and fungal strains known to cause UTI allowing laboratories to confidently diagnose patients in a timely manner, aiding with targeted treatments and helping to reduce risk of complications.
The Ongoing UTI Battle
Maternal UTI is a very common problem resulting in many fatalities and morbidities worldwide. It is crucial to identify and characterize these infections to limit the negative effects seen to both mothers and their children. Quick and efficient screening is paramount in the battle against bacteria to allow the prescription of targeted treatment. While antibiotics are often an effective weapon against UTIs, care should be taken when prescribing these treatments to pregnant women due to the potential adverse effects that have been reported. Furthermore, unnecessary treatments using antibiotics should be avoided at all costs due to the increasingly serious issue of antimicrobial resistance.
References
1.Eslami V, Belin S, Sany T, Ghavami V, Peyman N. The relationship of health literacy with preventative behaviours of urinary tract infection in pregnant women. Journal of Health Literacy. 2022;6(4):22-31. doi:https://doi.org/10.22038/jhl.2021.59768.1183
2.Bien J, Sokolova O, Bozko P. Role of Uropathogenic Escherichia coli Virulence Factors in Development of Urinary Tract Infection and Kidney Damage. International Journal of Nephrology. Published online 2012:1-15. doi:https://doi.org/10.1155/2012/681473
3.Werter DE, Kazemier BM, van Leeuwen E, et al. Diagnostic work-up of urinary tract infections in pregnancy: study protocol of a prospective cohort study. BMJ Open. 2022;12(9):e063813. doi:https://doi.org/10.1136/bmjopen-2022-063813
4.Balachandran L, Jacob L, Al Awadhi R, et al. Urinary Tract Infection in Pregnancy and Its Effects on Maternal and Perinatal Outcome: A Retrospective Study. Cureus. 2022;14(1). doi:https://doi.org/10.7759/cureus.21500
5.Bono MJ, Reygaert WC. Urinary Tract Infection. Nih.gov. Published 2018. https://www.ncbi.nlm.nih.gov/books/NBK470195/
6.World Health Organization. Preterm birth. Who.int. Published February 19, 2018. Accessed February 8, 2023. https://www.who.int/news-room/fact-sheets/detail/preterm-birth
7.Sinawe H, Casadesus D. Urine Culture. PubMed. Published 2021. https://www.ncbi.nlm.nih.gov/books/NBK557569/
8.Schrader SM, Botella H, Vaubourgeix J. Reframing antimicrobial resistance as a continuous spectrum of manifestations. Current Opinion in Microbiology. 2023;72:102259. doi:https://doi.org/10.1016/j.mib.2022.102259
9.Murray CJ, Ikuta KS, Sharara F, et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. The Lancet. 2022;399(10325):629-655. doi:https://doi.org/10.1016/S0140-6736(21)02724-0
10.Ali J, Rafiq QA, Ratcliffe E. Antimicrobial resistance mechanisms and potential synthetic treatments. Future Science OA. 2018;4(4):FSO290. doi:https://doi.org/10.4155/fsoa-2017-0109
11.Nelson DW, Moore JE, Rao JR. Antimicrobial resistance (AMR): significance to food quality and safety. Food Quality and Safety. 2019;3(1):15-22. doi:https://doi.org/10.1093/fqsafe/fyz003
12.Myers AL. Curbside Consultation in Pediatric Infectious Disease : 49 Clinical Questions. Slack; 2012:4.
13.Oie S, Kamiya A, Hironaga K, Koshiro A. Microbial contamination of enteral feeding solution and its prevention. American Journal of Infection Control. 1993;21(1):34-38. doi:https://doi.org/10.1016/0196-6553(93)90205-i
7. Sinawe H, Casadesus D. Urine Culture. PubMed. Published 2021. https://www.ncbi.nlm.nih.gov/books/NBK557569/
ABOUT RANDOX
NEWS
VACANCIES
OUR PEOPLE
Significant drop in veterinary antibiotics sales across Europe
Randox Food Diagnostics recently reported that the European Parliament has banned the use of antibiotics that are important for human medicine use on animals, and is prohibiting any antimicrobials in livestock without a vet prescription. The new legislation, that is to become law by 2022, states that antimicrobials cannot be used to improve performance or compensate for poor animal conditions.
The European Medicines Agency (EMA) have now documented a significant drop in overall veterinary antibiotic sales across Europe. The EMA recognise that the reduction highlights the efforts made by the European Union (EU) and various stakeholders, promoting prudent use of antibiotics in the animal sector and its positive impact. The reduction of antibiotic use in food-producing animals is a key pillar to the EUs One Health Action Plan against Antimicrobial Resistance (AMR), according to a report conducted by the University of Minnesota.
30 countries in total submitted data between 2011 and 2016. German antibiotic sales dropped by 58%. However, whilst the majority of countries saw a drop in sales, six countries reported an increase of more than 5%. Germany’s implementation of an antibiotic minimising programme has helped the country to minimise antibiotic use, by requiring farmers raising cattle, pigs, chickens, or turkeys to report the frequency of antibiotic treatment on their farm every 6 months. If animal treatment frequency is above the median of all farms, operators must evaluate their usage with a veterinarian.
This major step forward in public health has a direct impact on the environment and food. Randox Food Diagnostics recognise the importance of improving the global food safety chain, and continue to transform the landscape by developing high quality revolutionary screening products. Our reliable and economic testing methods enable the user to detect multiple drug and toxin residues from a single sample, including antimicrobials, growth promoting compounds, synthetic steroids, anthelmintics and coccidiostats. With an expanding range of 37 ELISAs, 21 multiplex screening arrays and 20 enzymatic/colourmetric reagents, our trusted solutions ensure that better science means safer food.
For any questions, email us directly at: info@randoxfooddiagnostics.com


