Whole pathogen multiplex controls to support testing for cold, cough and COVID
27 October 2020
Whole pathogen multiplex controls to support testing for cold, cough and COVID
Just in time for flu season, global diagnostics company Randox Laboratories has unveiled a range of whole pathogen multiplex controls that cover multiple viral and bacterial pathogens which cause respiratory disease.
Inclusive of SARS-CoV-2, Influenza A & B, and Respiratory Syncytial Virus (RSV), the Qnostics Multiplex Respiratory Pathogen Controls (RTX) facilitate diagnosis of the likes of cold and flu, and importantly, the differentiation of these respiratory diseases from COVID-19.
Using a control line that covers a range of respiratory pathogens in this way will allow laboratories to streamline their testing, consolidate QC, and ultimately save time, money and resource.
Lynsey Adams, Randox Quality Control Manager, explained;
“When the use of time and resources are critical such as in COVID-19 testing, the use of highly characterised controls enables laboratories to meet their daily QC needs and accreditation requirements.
“Accurate and reliable laboratory testing is an essential aspect of the management of COVID-19 and other respiratory diseases, so we are pleased to be able to offer these new controls which will crucially support the validation, verification and performance monitoring of molecular respiratory assays.”
The new RTX controls, which are whole pathogen for compatibility with the majority of commercial and in-house assays, are used to monitor the full testing process, from extraction to amplification and detection.
The Multiplex Respiratory Controls will support public health testing strategies during the incoming flu season and are suited for the test menus of most syndromic assays.
Lynsey continued;
“The Randox Qnostics Multiplex Respiratory Pathogen Controls are clinically relevant for full-process validation. Liquid frozen for user convenience and ease of use, the development of the controls using whole pathogen material ensures clinical relevance from extraction to amplification and detection.”
Randox Qnostics Multiplex Respiratory Pathogen Controls are manufactured to ISO 13485 standards and are in line with ISO 15189:2012 regulatory requirements.
Key Features and Benefits of the new RTX Controls:
- Whole pathogen controls – the controls contain the entire genome meaning they are compatible with the majority of commercial and in-house assays.
- Full process control – whole pathogen controls are the ideal material for full-process validation, monitoring the testing process from extraction to amplification and detection, to ensure ultimate quality assurance in laboratories.
- Highly characterised – Qnostics controls are quantified by digital PCR to ensure batch to batch reproducibility and are traceable to an internal reference preparation, to ensure metrological traceability of test results obtained by different diagnostic workflows.
- True Third Party – An independent, unbiased assessment of assay performance is ensured in line with ISO 15189:2012 regulatory requirements.
- Superior Manufacturing – Qnostics controls are manufactured under ISO 13485 guidelines to ensure quality and traceability.
- Liquid for Ease-of-Use – the controls are conveniently supplied in a liquid frozen format meaning there is no additional preparation or handling required.
For more information visit https://www.randox.com/molecular-infectious-disease-controls/respiratory-infection-testing/
For further enquiries please email marketing@randox.com
QNOSTICS
QCMD
CORONAVIRUS
Randox & Bosch Healthcare – Collaboratively Combating COVID-19
29th October 2020
Randox & Bosch Healthcare – Collaboratively Combating COVID-19
A game-changing partnership between Randox Laboratories and Bosch Healthcare Solutions continues to change the testing landscape and capabilities of both laboratory and non-laboratory settings in rapidly detecting COVID-19.
Providing clear and concise results, direct at the point of care, the Randox and Bosch Healthcare Solutions collaborative approach not only allows patients to take the recommended safety precautions without delay, but also provides a solution to both analyse and differentiate COVID-19 from other viral respiratory diseases. Multiple testing approaches with the same aim – to overcome COVID-19!
Combining science & technology, timing is of the essence in the fight against coronavirus. Together, we have combined efforts in successfully launching the Vivalytic – the all in one solution for molecular diagnostics consolidating nucleic acid extraction, polymerase chain reaction (PCR) and detection onto one small platform.
These efforts reflect a global partnership of both cooperation’s working together to deliver world-class diagnostic solutions, harnessing the power of innovation to improve health worldwide, providing an all in one approach minimising the spread of COVID-19 globally.
Launched after just six weeks at the beginning of the COVID-19 pandemic back in March 2020, the Viral Respiratory Infection array (VRI) set the benchmark for rapidly detecting SARS-CoV-2 (COVID-19) whilst simultaneously differentiating between nine other respiratory diseases, in under two and a half hours.
Proactively working together to combat the spread of COVID-19, Randox, Bosch Healthcare Solutions and R-Biopharm are proud to offer variable analysis strategies to detect SARS-CoV-2 (COVID-19). Capable of providing reliable results in just 39 minutes, with a sensitivity of 98% and specificity of 100%, the Vivalytic SARS-CoV-2 rapid test is currently among one of the fastest PCR tests worldwide.
Since the outbreak of COVID-19, we have significantly combined our efforts to provide a diverse range of testing solutions and approaches to meet the need of the rise in global cases. Our approaches have been utilised at all possible levels and together we open up a range of different testing strategies, with developments still underway with the upcoming release of the SARS-CoV-2 Pooling test.
The SARS-CoV-2 Pooling test is a rapid solution for the detection of SARS-CoV-2 (COVID-19) offering an accelerated decentral testing approach to effectively and efficiently monitor and detect viral infection from the offset. Capable of detecting SARS-CoV-2 (COVID-19) from up to five pooled samples simultaneously with a capacity to process more than 160 patient samples a day. The pooling could be done at the level of a ward, medical specialty, social bubble, or group of colleagues. It has potential for use in other settings, such as pre-operative screening, schools & universities, prisons, nursing homes, primary care, and large workplaces. Sample pooling allows more people to be tested quickly using fewer testing resources.
Partnering together, we continue to increase our testing capacity, enabling fully automated processing of patient samples to rapidly combat COVID-19.
Please contact marketing@randox.com for further information.
Randox in the media
Latest News
FAQs
Randox response to comments within Sunday Times article 18th October 2020

Randox response to comments within Sunday Times article 18th October:
Randox rejects comments made in the Sunday Times on 18th October, related to Randox’s contribution to the UK’s Covid-19 national testing programme, as misleading and inaccurate.
Randox have committed, through private investment, to the rapid growth of Covid-19 testing capacity within the UK. Indeed Randox are successfully operating at considerable scale and are currently providing well in excess of the testing capacity we have committed to the UK’s testing programme.
The vast majority of samples are reported within 24 hours of entering our laboratories.
Those samples that are found to be unsuitable for processing on receipt at our facilities, and are therefore voided, are not the responsibility of Randox. Such samples are voided when issues have arisen during sample collection and logistic processes, controlled by other parties.
It should also be noted that the proportion of samples found to be unsuitable on receipt at our facilities are both very low and comparable with all other laboratories across the programme.
Randox remain committed to supporting the UK’s Covid-19 testing programme and fully understand the importance of our work to the social and economic well being of the nation.
Press enquiries should be emailed to randox@newcenturymedia.co.uk
Randox in the media
Latest News
FAQs
Vivalytic | SARS-CoV-2 Pooling Test

Vivalytic | SARS-CoV-2 Pooling Test
–
Detecting SARS-CoV-2 (COVID-19)
Detecting SARS-CoV-2 (COVID-19) from up to 5 Pooled Samples Simultaneously
Clinical Significance
SARS-CoV-2 Pooling (COVID-19) is a rapid real time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care. This enables the patient to take the recommended safety precautions without delay. The pooling could be done at the level of a ward, medical speciality, social bubble, or group of colleagues. It has potential for use in other settings, such as pre-operative screening, schools and universities, prisons, nursing homes, primary care, and large workplaces. Sample pooling allows more people to be tested quickly using fewer testing resources.
In collaboration with Bosch, we are proud to release not only a rapid testing solution for the detection of SARS-CoV-2 (COVID-19) but an accelerated mass testing solution to effectively and efficiently monitor and detect viral infection from the offset with an aim of minimising the rise in infections globally. The new SARS-CoV-2 pooling test will allow users to test up to 160 samples a day and has sensitivity of 98% and a specificity of 100% – a world’s first!* Highly sensitive assays allow for laboratories to accurately detect low positive samples, enabling for effective identification of positive COVID-19 cases in a timely manner.
* Detailed information on the determination of analytical performance can be found in instructions for use provided with SARS-CoV-2 pooling kit.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab
Sample Volume: 150 μL per-patient sample (If
less than 5 patient samples, supplement the remaining
volume with eNAT solution)
Detection Method: Real-Time PCR
Time to result: 44 minutes
| Detectable Pathogens |
|---|
| SARS-CoV-2 (E gene sequence) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
SARS-CoV-2 Rapid Test
Vivalytic
Vivalytic Test Menu
EU Traffic Light System: How to get a COVID-19 PCR test before travelling

12 October 2020
EU Traffic Light System: How to get a COVID-19 PCR test before travelling
EU Traffic Light System: How to get a COVID-19 PCR test before travelling
As Covid-19 infection rates continue to rise in many parts of the world, an increasing number of countries are asking for a negative PCR test as a means of proof that travellers do not have the Covid-19 virus.
Whilst not yet a common requirement globally, it is understood that the EU could soon be turning to a Traffic Light System that will require pre-departure testing for individuals departing any of the 15 ‘red list’ countries – which includes the UK and could be extended to include Ireland.
Whilst there are currently a number of countries across the EU that do not require a negative COVID test for visitors from the UK, the implementation of this co-ordinated approach across the EU will place common requirements on all travellers from the UK, no matter which country they are visiting.
It could also be used in support of reduced quarantine in those countries which require it.
So – what is a PCR test?
PCR stands for Polymerase Chain Reaction and is a method of testing used to detect infectious disease, including COVID-19.
A PCR test is performed in a laboratory and is indicative of an individual’s COVID status at the time their sample is taken. It does not show previous infection.
Where can I get a COVID PCR test done?
PCR tests are available at Randox Health via an in-clinic appointment, or a home sample collection.
Are home tests accepted as proof of my COVID status?
It is important to note that many countries do not accept ‘home tests’ as proof of a negative COVID-19 result. Results must show that the test was performed in a certified laboratory.
Whilst Randox Health offers a home sample collection kit, all our testing is performed using PCR methods in our certified laboratories.
If the country you are travelling to requires a PCR test result, we’ve got you covered.
How do I know if I need a COVID PCR test?
You can check the entry requirements for your destination at gov.uk/foreign-travel-advice
Requirements are subject to change, so keep checking in the weeks and days leading up to your trip.
When do I take my sample for testing?
Your destination country will determine the time frame required for testing. For example, it may state that your sample needs to be taken and tested within 72 hours before departure / arrival. You cannot be swabbed before this time.
It is best to book / order your test in advance and take it as early as possible within your window, so that you allow for the maximum amount of time to get your test results back.
How long do I wait for my results?
Upon receipt of sample at Randox’s laboratories, you will receive your results within 24 hours.
Our rapid, accurate and reliable COVID-19 testing service will help get your trip off to a hassle-free start, with minimum disruption to your travel plans.
Visit https://www.randoxhealth.com/covid-travel-certificate/ to order your home sample collection kit or book your clinic appointment.
For further information please email info@randoxhealth.com or phone 0800 2545 130.
Want to know more about Randox?
Contact us or visit our homepage to view more.
Our COVID-19 Products and Services
HOME TEST
ANTIBODY TEST
TRAVEL CERTIFICATE
LABORATORY TOOLS
We Are Randox | How Courtney became a COVID-19 Scientist and Trainer
08 October 2020
We Are Randox | How Courtney became a COVID-19 Scientist and Trainer
In support of our new £30m COVID-19 laboratories, we are recruiting across Engineering, Manufacturing and Science.
For an idea of what it’s like to be part of the COVID-19 testing programme at Randox, we spoke to Senior Scientist and Trainer Dr Courtney Ward.
Courtney spoke to us about what a typical day in our laboratories looks like, the career path she took to her current role, and how it feels to be making a difference in the global fight against COVID-19.
Name: Dr Courtney Ward
Job Title: Senior COVID-19 Scientist and Trainer
Department: COVID-19 Laboratories
Give a brief outline of your career to date.
During my undergrad studies, I spent a year working on developing drug delivery technologies for a pharmaceutical company in England. Then during my PhD I worked in the Research Funding team at Cancer Research UK, and subsequently, following the completion of my PhD, at the Centre for Cancer Research and Cell Biology at Queen’s University Belfast.
My next move led me to Randox, to work on new ways to diagnose a range of diseases.
What was your favourite subject at school?
At school I always enjoyed science, and in particular biology. Since a child I have been fascinated with how the human body works and how different diseases can affect this.
Did you go on to further/higher education, if so what did you study and where?
I studied for eight years at Imperial College London. This included an undergraduate in Biochemistry, a Masters degree in Structural and Molecular Biology, and finally, a PhD in Structural Biology/Biophysics, for which I studied how bacteria affect human cells during infection.
How did you get into your area of work?
As I had previous experience in infectious disease testing, I moved this year into the Randox COVID-19 team to help respond to the urgency of the situation. As our testing capacity, and subsequently our staffing levels, have increased so extensively, I also use my experience to train our new scientists, which is something I really enjoy.
Is this what you always wanted to do?
I have always loved science and knew my career would be within this sector. I also enjoy teaching and training, so being a COVID-19 trainer has combined my two favourite roles into one which has been great.
Were there any particular essential qualifications or experience needed?
Scientists involved in COVID testing will be exposed to molecular testing techniques including DNA extraction, PCR techniques and Biochip technology, so experience in these areas is advantageous. There are also a number of positions – like accessioning or administration – that do not require a science background and we include all the training needed for these roles.
What are the main personal skills your job requires?
For roles in testing, the most important skills are concentration and attention to detail. We deal with 1000s of samples in a shift so it is crucial to manage each one carefully as the results are so important to each individual patient. You also need to be a team player, focused and able to take initiative.
What does a typical day entail?
A typical day in our COVID-19 laboratory begins with a handover from the previous shift and then we are assigned our roles for the day. You may spend your day preparing reagents using liquid handling robots, or you may be involved in organising samples along with their corresponding paperwork. It is a varied and exciting role, as things move very quickly in the world of COVID-19 testing.
What are the best and most challenging aspects of the job?
The best thing about working in the Randox COVID labs is knowing you are contributing to the national testing effort and therefore making a real difference. Working with a team is great and gives you the chance to meet a lot of new people for a lot of different backgrounds. I also really enjoy seeing new scientists improve in confidence during their training. Seeing them working well on shift gives me great sense job satisfaction.
The most challenging aspect of COVID testing can be the time pressure, as getting results out to the patient as fast and accurately as possible means teams must work seamlessly together. Similarly with training, we need to ensure we have enough staff to support our testing labs and so this can lead to a lot of new staff needing training which we need to work through quickly and efficiently.
Why is what you do important?
COVID-19 has had a huge impact on every aspect of our lives. To be involved in testing, which is absolutely crucial in identifying clusters of infection and reducing further spread, is so important to me and spurs me on to work to the absolute best of my ability.
What advice would you give anyone looking to follow a similar career path?
For me, making sure you take any opportunity to further your development is critical, be it the prospect of learning a new technique or method, or the chance to join the fight against COVID-19! I have always jumped at the chance to learn something new and this has set me in good stead for my current role as a trainer.
If you weren’t doing this what would you like to do?
If I weren’t involved in laboratory work, I would still carry on my love of science and training, by teaching science. I have always loved teaching, and I tutor in my spare time – to inspire the next generation to study and work in STEM subjects.
What is the one piece of advice you would give to yourself on your first day?
Take in as much information as possible – there will be a lot of it! Ask as many questions as you can, particularly if you are unsure of anything.
Describe your ideal day off.
Catching up on Real Housewives and taking my dog to the beach for a swim.
And finally, what’s the key to any successful job search?
Make sure you read the job description and tailor your CV to each role to which you apply. Make it easy for the employer to see how your skills and experience meet the criteria for the job.
We are delighted to have Courtney with us at Randox as part of our COVID-19 testing programme.
For current vacancies at Randox please visit randox.getgotjobs.co.uk
For more We Are Randox stories about our amazing colleagues, make sure to follow us on Facebook, Instagram and Twitter and follow the hashtag #WeAreRandox.
For further information please email recruitment@randox.com or phone 028 9442 2413.
Want to know more?
Contact us or visit our Randox Careers
Find out more about Randox Careers
News
Vacancies
Our Team
Staff Profiles
Vivalytic | Sexually Transmitted Infection Array

Vivalytic | Sexually Transmitted Infection Array
–
1 Sample | 1 Test | 10 Infections
Comprehensive Sexual Health Profile
Clinical Significance
The Sexually Transmitted Infection (STI) array is the broadest multiplex cartridge-based STI test on the market simultaneously detecting 7 bacterial, 2 viral and 1 protozoan infection for a comprehensive sexual health profile.
Globally, each day over 1 million people are exposed to an STI, many of who will not know they have an infection as many STI’s are asymptomatic whilst some individuals will display similar or overlapping symptoms, therefore co-infections may remain undiagnosed. Designed to offer a complete sexual health profile with an aim of prevention and control, the Vivalytic STI array can be used to diagnose existing infections whilst any identifying co-infections.
To find out more about the global economic burden of STIs and the impact of the COVID-19 pandemic on sexual health, download our most recent whitepaper that presents issues such as AMR, the development of super-gonorrhoea, and the challenges faced in a social and public concept due to the COVID-19 pandemic.
Features
Sample Type: Swab or Urine (eNAT, Roche COBAS medium, or PBS)
Sample Volume: 300 μl
Detection Method: Randox Biochip Technology (end-point PCR)
Time to result: 2 hours 20 minutes
| Detectable Infections | |
|---|---|
| Chlamydia trachomatis (CT) | Herpes simplex virus 1 (HSV-1) |
| Neisseria gonorrhoeae (NG) | Herpes simplex virus 2 (HSV-2) |
| Trichomonas vaginalis (TV) | Haemophilus ducreyi (HD) |
| Mycoplasma genitalium (MG) | Mycoplasma hominis (MH) |
| Treponema pallidum (Syphilis) (TP) | Ureaplasma urealyticum (UU) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
Vivalytic Test Cartridges
Vivalytic
Vivalytic Test Menu
New 39-minute COVID test available on Randox-Bosch Vivalytic
02 October 2020
New 39-minute COVID test available on Randox-Bosch Vivalytic
- The world’s fastest PCR based SARS-CoV-2 test for the point of care delivers reliable results in 39 minutes.
- Has a sensitivity of 98 percent and a specificity of 100 percent.
- Simultaneous testing of five people with one cartridge by pooling will be available from early October.
- Work is in progress to further reduce time to result.
A rapid new coronavirus test, which provides results for Covid-19 in just 39 minutes, is now available on the Vivalytic, a point of care platform brought to market by Randox Laboratories and Bosch.
The test for detection of the SARS-CoV-2 pathogen, is currently the fastest PCR test (the gold standard of test methods) worldwide, and is predestined for decentralized use in mobile test centres at service stations or in airports, so that people who take the test can obtain a reliable result while at the testing site.
Available now in Europe, the CE-approved test, which has a sensitivity of 98 percent and a specificity of 100 percent, helps avoid time in quarantine, relieve laboratories, and make travel and work safer again.
“Rapid and accurate testing plays a crucial role in identifying cases of Covid-19 – to contain any outbreaks and limit the spread of the virus,” says Dr. Heather McMillan, Molecular R&D Manager at Randox Biosciences.
“This new rapid test will be a game-changer in the coronavirus testing landscape by allowing patients to receive their results at the point of care faster than ever before.”
Randox and Bosch launched the first rapid test for the Vivalytic analyser at the end of March, after just six weeks’ development.
As a multiplex test, it simultaneously checks samples for the SARS-CoV-2 virus as well as nine other respiratory diseases in two and a half hours, whereas the new accelerated test is exclusively for SARS-CoV-2.
“With our different coronavirus tests and variable analysis strategies, we open up a range of testing scenarios with a Vivalytic device – from screening all the way to supporting differential diagnosis for similar symptoms,” says Marc Meier, president of Bosch Healthcare Solutions GmbH.
And development work for Covid tests on the Vivalytic is ongoing: as of early October 2020, by pooling samples together it will be possible to simultaneously evaluate five samples in one test cartridge and at a comparable speed – a world first.
This will increase available testing capacity, by enabling fully automated processing of more than 160 samples a day using a Vivalytic device.
Key Benefits of SARS-CoV-2 test on Vivalytic point of care platform
The advantages of the rapid SARS-CoV-2 test on Vivalytic lie not only in speedy analysis, but also in ease of use. A sample is taken from the nose or throat using a swab, and placed in the test cartridge. Then the cartridge, which contains all the reagents required for the test, is inserted into the Vivalytic device for automated analysis.
- Turnaround time of 39 mins from sample entry to result.
- The SARS-CoV-2 rapid test has recently received CE marking.
- The SARS-CoV-2 pooling test can run up to 5 samples on-board one single cartridge.
- Easy 4-step user-friendly process from sample entry to result. Minimal training required.
- Detection from real-time PCR from Nasopharyngeal and/or Oropharyngeal swab.
- Suitable for use in any laboratory and non-laboratory settings.
The development of the new Vivalytic PCR singleplex test is part of a research and development project funded by the German Federal Ministry of Education and Research (BMBF).
For more information please contact marketing@randox.com
RESEARCH
BIOPHARMA
CLINICAL LAB
BIOREAGENTS
Vivalytic | Test Cartridges

Vivalytic | Cartridges
–
Powered by Biochip Technology
Simple & Accurate POC Molecular Diagnostics
Vivalytic cartridges are compact, technologically advanced Molecular Diagnostic tests utilising micro-fluidics to enable simple and accurate diagnostic testing. Vivalytic cartridges are powered by a variety of technologies, dependent upon the test application. High-Plex and Low-Plex tests can be analysed on the Vivalytic. High-Plex tests utilise Randox patented Biochip Array Technology, enabling end-point qualitative PCR and providing multiple test results from each sample. Low-Plex tests are based on a variety of detection methods including real-time qualitative PCR and melting curve analysis.
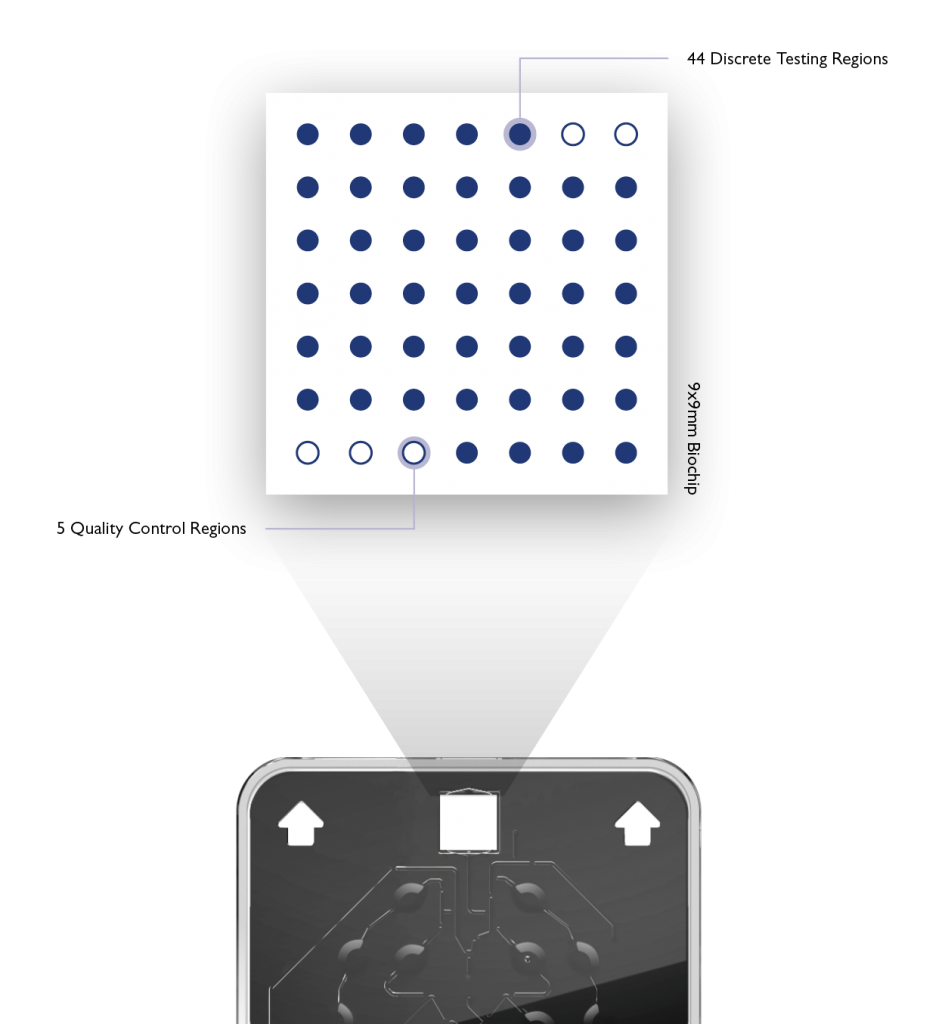
Randox patented Biochip Technology allows simultaneous detection of multiple targets from a single patient sample. The biochip detection system is based on a chemiluminescent signal, this is the emission of light, without heat, as a result of a chemical reaction. Each biochip is prefabricated with spatially discrete testing regions (DTR’s).
Each DTR represents an individual test. Each DTR can be occupied with oligonucleotides specific to a pathogen or target of interest. The High-Plex capabilities of Biochip Technology eliminates the need to run multiple time consuming and sample intensive assays.
An enzyme is used to catalyse the chemical reaction of the biochip which generates the chemiluminescent signal. The light emitted from the chemiluminescent reaction that takes place in each DTR is simultaneously detected and quantified using a Charge – Coupled Device (CCD) Camera. This CCD Camera simultaneously records the light emission from all the DTRs on each biochip. The Vivalytic automatically generates a result report for all targets.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
SARS-CoV-2 Rapid Test
VRI Array
Vivalytic
Vivalytic Test Menu
Vivalytic | SARS-CoV-2 Rapid 39 Minute Test

Vivalytic | SARS-CoV-2 Rapid 39 Minute Test
–
Detecting SARS-CoV-2 (COVID-19)
Rapidly Detecting SARS-CoV-2 (COVID-19)
Clinical Significance
SARS-CoV-2 (COVID-19) is a rapid real time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care. This enables the patient to take the recommended safety precautions without delay.
In collaboration with Bosch, we are proud to release not only a rapid testing solution for the detection of SARS-CoV-2 (COVID-19) but an accelerated mass testing solution to effectively and efficiently monitor and detect viral infection from the offset with an aim of minimising the rise in infections globally. The new SARS-CoV-2 pooling test will allow users to test up to 160 samples a day and has sensitivity of 98% and a specificity of 100% – a world’s first!*
* Detailed information on the determination of analytical performance can be found in instructions for use provided with SARS-CoV-2 kit.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab (eNAT)
Sample Volume: 300 μl
Detection Method: Real-Time PCR
Time to result: 39 minutes
| Detectable Virus |
|---|
| SARS-CoV-2 (E gene sequence) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products












