Prime Minister Boris Johnson congratulates Randox

Randox in the media
Latest News
FAQs
Matt Hancock pays tribute to Randox staff in House of Commons

Randox in the media
Latest News
FAQs
Randox response to Sunday Times Article 20 September 2020

Randox in the media
Latest News
FAQs
Vacancies available in support of the COVID-19 National Testing Programme

17 September 2020
Vacancies available in support of the COVID-19 National Testing Programme
Randox response to Sunday Times Article 13 September 2020

Randox in the media
Latest News
FAQs
Vivalytic | The All in One Molecular Solution

Vivalytic | All In One Molecular Solution
–
Making a Point to Care
Molecular Diagnostics at the Point of Care
Consolidate the Full Molecular Workflow
Supporting Team GB through the Paris Olympics
The Vivalytic has supported Team GB by providing comprehensive testing for a wide range of respiratory infections from a single sample. This allows for targeted management of athletes’ health, ensuring precise treatment and quick recovery.
Regular and rapid testing with the Vivalytic helped maintain athletes in peak physical and mental condition by ensuring infection was detected and treated at the earliest opportunity. Check out the video to the right to learn more about how Vivalytic helped prepare and support Team GB.
“It’s important we know the pathogen driving the disease process to be able to target management appropriately“
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
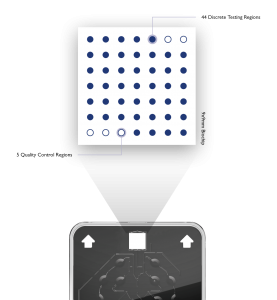
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Related Products
VRI Array
SARS-CoV-2 Rapid Test
SARS-CoV-2 Pooling Test
Vivalytic Test Menu
Randox selected to screen travellers to the UAE for Covid-19
We Are Randox | Staff Newsletter January – July 2020 Edition
Staff Newsletter January - July 2020 Edition
We are delighted to be able to share with you the January – July 2020 edition of our We Are Randox staff newsletter.
Click here to view our news from 2020 so far – including of course our vital work in testing for COVID-19, but also a range of staff announcements including engagements, births and retirements.
** Please note that this newsletter works most efficiently in your Google Chrome browser**
Dr Peter FitzGerald: “Nothing will ever be the same again.”

Jaffe Creatinine Assay
Reagent | Creatinine (Jaffe)
A Marker of GFR Function
Benefits of the Randox Jaffe Creatinine Assay
Excellent precision
The Randox Jaffe creatinine assay displayed a within run precision of < 4.0% CV.
Exceptional correlation
The Randox Jaffe creatinine assay displayed a correlation coefficient of at least r=0.99 when compared to commercially available methods.
Liquid ready-to-use
The Randox Jaffe creatinine assay is available in a liquid ready-to-use format for convenience and ease-of-use.
Calibrator and controls available
Calibrator and controls available offering a complete testing package.
Applications available
Applications available detailing instrument-specific settings for the convenient use of the Randox Jaffe creatinine assay on a variety of clinical chemistry analysers.
Ordering Information
| Cat No | Size | ||||
|---|---|---|---|---|---|
| CR510 | 1 x 200ml (S)(L) | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR3814 | R1 6 x 51ml (L) R2 3 x 28ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR8022 | R1 6 x 68ml (L) R2 6 x 20ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| CR8316 | R1 4 x 20ml (L) R2 4 x 7ml | Enquire | Kit Insert Request | MSDS | Buy Online |
| (L) Indicates liquid option (S) Indicates standard included in kit | |||||
Instrument Specific Applications (ISA’s) are available for a wide range of biochemistry analysers. Contact us to enquire about your specific analyser.
More useful information
Creatinine is the end-product of muscle catabolism of creatine. In humans, creatinine production is relatively stable, but mainly depends on muscles mass. Consequently, any physiological changes in muscle mass will cause a variation in the creatinine pool independently of GFR changes. Creatinine is freely filtered by the glomerulus at a constant rate with 10% to 40% secreted by the tubules 1.
According to the National Institutes of health, the overall prevalence of chronic kidney disease (CKD) is approximately 14% 2. Creatinine is the most commonly utilised assay in the assessment of renal function 3. The National Kidney Disease Education Program recommends calculating GFR from SCr. Creatinine measurements are useful in the monitoring of disease progression, with the diagnosis of renal failure when SCr levels are greater than the upper normal interval 4.
Creatinine measurements are useful in the diagnosis and monitoring of diabetic nephropathy, the leading cause of kidney disease in patients commencing renal replacement therapy, affecting 40% of diabetics (type 1 and type 2) 5. The RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) study risk score for end-stage renal disease (ESRD) emphasizes the importance of the identification of elevated SCr, alongside other renal markers, in the prediction of end-stage renal disease (ESRD) development in patients with type 2 diabetes mellitus (T2DM) and nephropathy 6.
Acute kidney injury (AKI) is a common complication in COVID-19 patients 7. The analysis of creatinine in COVID-19 patients on hospital admission and after 2 to 4 days highlighted impaired renal function and is the leading cause of death in these patients 8. The National Institute of Care Excellence (NICE), have set out four guidelines for acute kidney injury in hospitalised suspected or confirmed COVID-19 patients and highlights the importance of creatinine testing 9.
Clinical Chemistry Calibrator
Clinical Chemistry Controls
Clinical Chemistry EQA
References
[2] Gounden V, Bhatt H, Jialal I. Renal Function Tests. Treasure Island: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed 24 July 2020).
[6] Dabla PK. Renal function in diabetic nephropathy. World Journal of Diabetes 2010; 1(2): 48-56
[7] Mahmoudi H, Alikhani MY, Taheri NM, Behzadi A. Assessment of changes in blood urea and creatinine levels in patients with coronavirus disease 2019 (COVID-19). v 2020: https://www.researchsquare.com/article/rs-25164/v1 (accessed 16 July 2020)
[9] National Institute of Care Excellence (NICE). COVID-19 rapid guideline: acute kidney injury in hospital. https://www.nice.org.uk/guidance/ng175/chapter/4-Assessing-for-AKI-in-patients-with-suspected-or-confirmed- COVID-19 (accessed 16 July 2020).