Randox and Cormay Diagnostics Partner to Provide World-Leading EQA Programmes
Randox and Cormay Diagnostics Partner to Provide World-Leading EQA Programmes
Cormay Diagnostics and Randox Partner
Providing World-Leading Quality Assessment Programmes to Laboratories Worldwide
Cormay Diagnostics, a leader in diagnostic solutions, and Randox International Quality Assessment Scheme (RIQAS), a market leader in external quality assessment, have announced a strategic partnership to deliver world-class External Quality Assessment (EQA) programmes to laboratories across the globe.
The collaboration brings together the extensive expertise and experience of both companies to offer laboratories a comprehensive range of high-quality EQA schemes designed to enhance the quality and accuracy of laboratory testing worldwide. The partnership will allow Cormay Diagnostics to distribute and promote the RIQAS portfolio.
Supporting the shared mission of both Cormay Diagnostics and Randox to equip clinical laboratories of all sizes and budgets with cutting-edge technology and high-performance solutions. Helping all laboratories to meet regulatory requirements while boosting confidence in the accuracy of patient test results.

Cormay Diagnostics has over four decades of experience in manufacturing high-quality diagnostic reagents and providing laboratory solutions. The
company’s mission is to empower laboratories with the tools they need to ensure accurate, efficient, and reliable testing for healthcare professionals and patients worldwide.
Learn more: cormaydiagnostics.com

Founded in 1989, RIQAS is the worlds largest international External Quality Assessment Scheme with more than 76,000 laboratory participants spanning over 139 countries. With a product portfolio spanning 38 programmes, RIQAS offers participants a comprehensive yet cost effective solution to meeting regulatory requirements and increased confidence in test system accuracy.
Learn more: randox.com/riqas-external-quality-assessment
The Randox Grand National 2025 Trophy
The Randox Grand National 2025 Trophy
Randox is delighted to present the winner’s trophy for the Randox Grand National 2025. Expertly crafted by renowned Liverpudlian jewellery brand Boodles, this year’s trophy embodies the deep connection between Randox and the world’s most prestigious steeplechase, held annually at Aintree Racecourse. Inspired by Randox’s pioneering Biochip technology, the trophy’s striking design symbolises innovation, scientific advancement, and human endeavour. Subtle details within the craftsmanship also pay tribute to Aintree, seamlessly weaving elements of the iconic racecourse into its design.
Crafted with Purpose
The 2025 Randox Grand National Trophy is a masterpiece of design, seamlessly blending scientific innovation with the rich heritage of the world’s greatest steeplechase. The trophy prominently features a molecular structure and a DNA helix, represented by sixteen spheres and bars, mirroring the sixteen jumps of the Grand National course.
Encircling this intricate design is a stylised horseshoe, honouring the exceptional horses at the heart of this historic event and reflecting Randox’s deep-rooted passion for the sport. The trophy’s base, crafted from green malachite, is crowned with a Biochip-inspired design, inlaid with diamond discs. This element not only pays homage to Randox’s groundbreaking diagnostic technology but also evokes the tradition of incorporating precious gemstones into Grand National trophies throughout history.
Randox Founder and Managing Director, Dr. Peter FitzGerald, a passionate horseman himself, shared his thoughts on this year’s trophy:
“This year’s Randox Grand National Trophy, crafted by Boodles, is a stunning fusion of tradition and innovation. Its design, inspired by both Randox’s advancements in preventative healthcare and the rich legacy of the Grand National, is a fitting tribute to this world-renowned race. We are proud to collaborate with Boodles to celebrate excellence, heritage, and our shared commitment to pushing boundaries.”
Speaking on behalf of The Jockey Club, Dickon White, Regional Director for the West and Aintree Racecourse, stated:
“The Randox Grand National Trophy is a symbol of prestige, achievement, and history, and this year’s design by Boodles beautifully reflects these qualities. Their deep-rooted connection to both artistry and equestrian heritage makes them the perfect partner to create such an iconic piece. The trophy seamlessly blends Randox’s pioneering spirit with the enduring legacy of the Randox Grand National, resulting in a design that is both contemporary and timeless.”
A Return to Legacy
In recent years, Randox has designed the Grand National trophies in-house. However, for 2025, the honour has been entrusted once again to Boodles, a prestigious jeweller with a rich history of crafting Grand National trophies. This collaboration is driven by a shared passion for horse racing between Randox, Boodles, and The Jockey Club. Reflecting on this return to tradition, Michael Wainwright, Chairman of Boodles, shared his thoughts:
“Boodles is delighted to be partnering with Randox to design the 2025 Randox Grand National trophy. This collaboration is a wonderful opportunity to celebrate both the rich heritage of the Grand National and our longstanding connection to Aintree, with our company’s roots tracing back to 1798 in Liverpool, just a stone’s throw from the racecourse. We are honoured to play a part in commemorating this world-famous event with Randox.”
The Randox Grand National takes place 3rd – 5th April, 2025 at Aintree Racecourse.
Learn more here about the 3-day event here: The Grand National 2025 | 3-5 April 2025 | Aintree Racecourse
Acusera 24·7 and Data Innovations – Automated QC Data Upload

Automated QC Data Upload to Acusera 24·7
Now Available via Instrument Manager™ from Data Innovations
Randox Laboratories in conjunction with Data Innovations are pleased to announce the launch of a new driver that enables seamless, automated QC data upload via API from any instrument connected to Instrument Manager (IM) directly into Acusera 24.7. This new feature eliminates the need for manual data entry, reduces transcription errors and improves laboratory efficiency.
What you need to know
Uni-Directional License – Anyone wishing to avail of automated QC data upload to Acusera 24.7 will need a uni-directional license for Instrument Manager. A new license can be requested directly from a Data Innovations representative, or an existing license previously used for an analyser may be re-purposed.
Driver Download – Once a license has been secured, you will be required to download the Acusera 24.7 Driver from the MY DI Community website – https://datainnovations.my.site.com/s/drivers. To locate the driver simply select Randox from the Manufacturer drop down menu and then select Acusera 24.7 from the list of available drivers.
Configuration – Once the driver has been downloaded, you will receive help files that document the process to be followed. The same steps are to be followed as with connecting any instrument or analyser to Instrument Manager.
Once the driver has been successfully set up, Instrument Manager will automatically send QC data to Acusera 24.7, removing the need for manual data entry.
Key Considerations
- You must have have an Acusera 24.7 license and configured your Acusera 24.7 account before connecting to Instrument manager.
This new capability allows laboratories to optimise workflows, reduce errors, and streamline QC data management.
About Data Innovations
Data Innovations is a global software company that is passionate about excellence in patient care. Through innovative solutions and world-class service, they enable enterprise management of hospitals and independent laboratories. Founded in 1989 with headquarters in Vermont, Data Innovations serves more than 6,000 hospitals and laboratories in 80+ countries.
What is Instrument Manager?
Laboratory Information Management Systems (LIMS) by Data Innovations LLC
Instrument Manager™ (IM) is a truly vendor-neutral middleware platform that allows for the connection of any clinical lab instrument to any Lab Information System (LIS), with a library of over 1,000 instrument drivers. IM offers a suite of lab enablement solutions spanning productivity, quality, analytics, performance and reliability.
Labs using IM auto verification with time-saving workflows are able to achieve quicker turnaround times and increased productivity. IM quality assurance programs ensure that all connected instruments deliver the most accurate possible test results.
Randox named as Company of the year
Randox tops the charts in Belfast Telegraph Top 100 Companies
Randox is the largest blood-sciences healthcare diagnostics company from the UK and Ireland. Established in 1982 by managing director Dr Peter FitzGerald, Randox manufactures more than four billion tests per year and has a global sales and distribution network supplying product to 145 countries. Randox estimates more than 400 million people, around 5% of the world’s population, receive a diagnosis involving Randox products each year. Key facilities are located at multiple sites within Northern Ireland, in Co Donegal, in West Virginia in the United States and in Bengaluru, India.
As a privately-owned company Randox invests heavily in research and development, committing up to 25% of turnover for the development of new diagnostic products. Recent testing developments include: Type 1 Diabetes Genetic Risk Score; an advanced PSA test to improve the predictive potential of PSA alone for prostate cancer; predictive bladder cancer testing panels; risk assessment for post operative acute kidney injury and chronic kidney disease; and wide-ranging quality control and external quality assurance capabilities to ensure the accuracy and reliability of laboratory results.
Randox’s innovative approach to R&D has allowed the development of allied capabilities outside the immediate world of human healthcare, including leading testing capabilities in the veterinary field, for toxicology and for the detection of harmful drug residues and pathogens in food.
Research and development programmes are not limited to the development of tests, but also includes the advanced analyser systems to process samples efficiently. This requires integral, advanced capabilities in electrical and mechanical engineering, optics, robotics and software engineering.
Over £350m of investment has supported the development of a unique testing system to allow multiple tests to be run simultaneously on a single sample – the Randox Biochip. This 9mm x 9mm platform, which allows up to 49 tests to be run simultaneously, greatly increases the diagnostic power available to clinicians to enable earlier, more accurate diagnosis and improved patient outcomes. Initially focused on proteomics, Randox has also greatly increased its genetic testing capabilities to increase predictive capabilities.
In order to support the model of diagnostically-led healthcare, Randox has developed the Randox Health brand, to provide public access to comprehensive testing. The aim is to empower the consumer and to enable a preventative approach to healthcare – rather than rely upon the treatment of illness. Well over 20 clinics are now available across the UK and Ireland, including in partnership within John Lewis stores.
At the outset of the Covid-19 pandemic Randox was ideally placed to support the UK’s requirement for a national Covid-19 testing programme. By mid-February 2020 Randox were amongst the global frontrunners in developing a Covid-19 test and had developed home sample collection and reporting capabilities. The company was engaged by Government from mid-March 2020 to support the UK’s national testing programme – at that time the complete NHS testing capability for Covid-19 was reported as 2,400 tests per day.
Rising to meet this unprecedented national demand markedly increased company turnover and profits. A post-pandemic assessment by consultants OCO Global concluded that Randox’s activities during the pandemic averted over 3,100 deaths, prevented 14,100 hospitalizations and enabled a £8.3bn contribution to the UK’s GDP.
Exiting from the pandemic and reverting to sector norms with a strong balance sheet, Randox is investing heavily in upgrading the company’s infrastructure and R&D programmes. Randox is also investing significant resources in expanding the business-to-consumer diagnostic offer under the Randox Health brand – with clinics in the UK and Ireland, and US.
Noting the pressures upon all healthcare systems, and a need to transition from ‘illness management’ to more sustainable ‘prevention’ models, Randox is investing heavily for the future. Its aim is to ensure it is well positioned to support and enable healthcare transformation through improved diagnostics, to both increase system efficiencies and improve patient outcomes.


Joe Kennedy III, US special envoy for economic affairs visited Randox Science Park in Antrim last week to listen for presentations on Randox Laboratories MultiSTAT and RABTA analysers. He wrote in a foreword to the Belfast Telegraph article, “I congratulate everyone who has contributed to the success of Northern Ireland’s Top 100 Companies. “From globally-recognised brands to indigenous firms, each company reflects the wide diversity of employers who generate prosperity and opportunity right across Northern Ireland.”
Randox managing director Dr. Peter FitzGerald said it welcomed the accolade of being named number one company.
“These results reflect the skills, commitment and agility of our people, and the capability of our unique technologies.
“As a team we were also able to call upon 40 years of diagnostic experience to meet the exceptional demands of Covid-19, while simultaneously supporting our established global customer base of healthcare laboratories. Crucial to this was our long term commitment to research and development which has always been a cornerstone of our business philosophy.
“Randox is committed to enhancing healthcare through more sensitive and accurate diagnostics to enable the prevention of disease and improve healthcare outcomes, whilst reducing the burden on healthcare services.
“Our profits will continue to be directed towards enabling our infrastructure and research and development, as well as accelerating better healthcare models and improving direct public access through our Randox Health clinics. Our ambition is to enable better health outcomes for all and we are committed to that goal.”
PCR rapid tests for Candida auris for Vivalytic by Bosch now available
World’s first fully automated PCR test for detection of the multidrug-resistant fungus at the point of care.
-
Vivalytic test detects Candida auris in under an hour at the point of care, making it the world’s first test suitable for screenings.
-
Candida auris infection rates are on the rise in Germany and can cause severe infections, for example in the bloodstream (sepsis).
-
Hospitals can use the new screening test to detect colonization, allowing them to implement measures to contain outbreaks.
Bosch Healthcare solutions has developed a PCR test for detecting Candida auris (C. auris) and on the Vivalytic platform. The test is a global innovation now available for order from distribution partners including Randox Laboratories Ltd. and R-Biopharm. This test enables the fully automated detection of the frequently multi-resistant fungus in less than an hour at the point of care. The rapid testing capability also makes it suitable for carrying out screenings when necessary. In contrast, traditional culture tests in centralized laboratories require one to three days, delaying diagnosis and the initiation of targeted treatment. “Considering the heightened risk of severe progression in individuals with pre-existing conditions, we have developed a new test that enables clinics to respond more swiftly,” states Marc Meier, managing director of Bosch Healthcare Solutions. Patients with compromised immune systems, such as those in intensive care, individuals with serious underlying conditions such as diabetes, or those who are immunosuppressed due to cancer or HIV, as well as patients about to undergo invasive surgery, face a heightened risk of active infection with C. auris. The mortality rate for C. auris infections ranges from 30 to 72 percent.
“Candida auris can be transmitted from person to person through contact and contaminated surfaces. When this fungus presents, rapid detection is therefore rapid detection is therefore paramount to enable implementation of effective control and prevention strategies,” says Dr. med. Alexander Maximillian Aldejohann, deputy head of the Würzburg Laboratory at the National Reference Center for Invasive Fungal Infections (NRZMyk.) Since July 2023, Germany has implemented a limited statutory reporting requirement under the Infection Protection Act. Aldejohann is in favor of extending this reporting obligation: “The fungus has the capacity to rapidly develop resistance to many common antifungal agents coupled with the ability to survive for a relatively long time on surfaces. This high so-called tenacity also increases the risk of outbreaks that are difficult to contain.”
Increasing spread of Candida auris
C. auris is spreading globally. In some states in the U.S, the annual incidence rate has been shown to increase by a factor of 2 to 3. The Robert Koch Institute (RKI) also drew attention to a rise in cases within Germany during the past year in the Epidemiological Bulletin at the at the beginning of May. The RKI points out that in specific areas screening could be beneficial. In the U.S, the annual case count has in the meantime reached the thousands. The Centres for Disease Control and Prevention (CDC) already consider the screening of patients, visitors, and staff for C. auris as a crucial strategy to curb its spread in healthcare settings. While the fungus is harmless for healthy individuals, it can it can lead to severe nosocomial infections, i.e. infections acquired in hospitals or other healthcare environments, in patients at high risk and, if the fungus enters the bloodstream, can trigger sepsis.
Easy handling, rapid detection The Vivalytic Analyser enables effortless testing directly at the point of care: The sample is placed into the test cartridge, which already contains all necessary reagents. The cartridge is then inserted into the Vivalytic Analyser for automated processing. Healthcare professionals require only minimal training to use the system, and the fully automated process significantly lowers the risk of infection. The Vivalytic Analyser thus facilitates rapid and precise diagnostics in PCR quality, bypassing the frequently lengthy process through a central laboratory. Bosch Healthcare Solutions is expecting CE certification for the Vivalytic C. auris test soon.
For More Information Please Contact:
Martin Conway, Phone: +44 (0) 28 9442 2413
E-mail: martin.conway@randox.co
Dementia Action Week 2024

Dementia Action Week 2024 (13th – 20th May)
The term Dementia describes the different brain disorders that trigger a loss of brain function. These conditions are all usually progressive and eventually severe. Alzheimer’s Disease is the most common type of dementia, affecting 62 per cent of all those diagnosed.
Dementia is a general term for loss of memory, language, problem-solving and other thinking abilities that are severe enough to interfere with daily life. Common symptoms include memory loss, confusion, and speech problems. Early warning signs may also include finding it difficult to follow conversations, or programs on TV, forgetting names of friends, or everyday objects and feeling confused even in a familiar environment.
Mainly affecting older people, after the age of 65, the likelihood of developing dementia roughly doubles every five years – however, for some dementia can develop earlier, presenting different issues for the person affected, their carer and their family. There is also a considerable economic cost associated with the disease estimated at £23 billion a year, which is predicted to triple by 2040. This is more than the cost of cancer, heart disease, and stroke.
At Randox, we recognise the importance in diagnosing dementia early. Through our Randox Alzheimer’s Disease Array which can be used for Rapid Identification of Alzheimer’s Disease Risk. Randox’s Alzheimer’s Disease Risk Array can be used for the direct determination of ApoE4 status from plasma, eliminating the need for genetic testing, assisting in clinical research and personalised medicine strategies. At Randox, we believe the importance of measuring ApoE4 protein expression in plasma is the way forward to screen those individuals at increased risk of Alzheimer Disease, as new beta amyloid-targeting therapies for this condition are being expected.
Race Against Dementia have been the partner charity for the Randox Grand National Festival over the past two years, working alongside this charity which was founded by three-times Formula One World Champion, Sir Jackie Stewart, with the aim of funding much needed pioneering research into the prevention and cure of dementia.
For further information about the Randox Alzheimer’s Array please email info@randoxbiosciences.com
Growth through medical technology: Randox and Bosch invest heavily in the Vivalytic analysis platform
-
New partnership for Vivalytic analysis platform: Bosch and Randox
-
Laboratories Ltd. to invest 150 million euros in research, development, and
-
distribution
-
Strategic growth field: point-of-care molecular diagnostics expected to
-
become a future market worth billions
-
Growth with fully automated laboratory diagnostics: the two companies aim to
-
achieve sales in the nine-figure range by 2030.
-
Smartphone-sized laboratory: sepsis IVD grade test based on cutting-edge
-
BioMEMS technology is a joint development objective.
With many diseases, every minute counts, and determining whether a patient is presenting the symptoms of a simple cold, the flu, or something as severe as life-threatening meningitis is usually only possible after time-consuming and expensive
laboratory diagnostics. With its Vivalytic analysis platform, Bosch has set itself the goal of making fast and highly precise diagnostics accessible at the point of care – and aims to use molecular diagnostics to become a leading provider in the
market by 2030. To achieve this, Bosch has now agreed on a strategic partnership with Randox Laboratories Ltd., a leading diagnostic and medical technology company. The two companies will invest around 150 million euros in joint research, development, and sales activities for new tests for the Vivalytic analysis platform provided by Bosch Healthcare. One goal is the development of a sepsis IVD1 grade test that will be the first to feature highly innovative and novel BioMEMS technology.
Bosch has defined medical technology as a strategic growth field. Point-of-care molecular diagnostics is expected to become a future market worth billions. And with its Vivalytic analysis platform, Bosch aims to achieve a leading position in this market. “With cutting-edge technology from our own labs and our own production lines, we want to grow long-term together with partners in the field of precision diagnostics,” says Stefan Hartung, chairman of the Bosch board of management. “Here, our medical technology can draw considerable benefit from our diversification, from our expertise, from the groundwork we have done in automation, miniaturization, molecular diagnostics, and from our experience in microchip development and manufacturing,” Hartung adds. “We are investing long-term in an exciting high-tech growth field and continuously developing it together with partners. With technology ‘Invented for life’, we can relieve the burden on medical professionals and help make the diagnose and treatment of disease faster”.
Shared growth: decentralized diagnostics at the point of care
Bosch Healthcare Solutions and Randox are now joining forces in an attempt to accelerate the development and market launch of new tests and to make distribution channels more efficient. The partnership is set to run for more than ten years. With Vivalytic, the two companies aim to achieve sales in the medium nine-figure range by 2030. “Globally, healthcare is moving toward decentralized and personalized diagnostics, that enable rapid interventions and individual treatment plans,” says Marc Meier, managing director of Bosch Healthcare
Solutions GmbH. “With our partner Randox, we want to further expand the test portfolio of our Vivalytic analysis device. Our fully automated molecular diagnostics PCR tests provide clarity directly at the site of sample collection,
shorten waiting times, and take the strain off the healthcare system,” Meier adds. The two partners are a good fit: Bosch can contribute its technology and manufacturing expertise across the fields of molecular diagnostics, microchip
development and manufacturing, and miniaturization. The universal Vivalytic platform for molecular diagnostics was developed in over ten years by Bosch researchers and brought to market maturity by Bosch Healthcare Solutions. Randox has 40 years of experience in the design and development of highly sensitive IVD tests performed on a variety of technologies, including microfluidic platforms. In combination with the company’s extensive market knowledge and global sales and distribution network, this adds up to considerable opportunities for growth. “Randox has always been committed to improving health worldwide and sees the need to invest in research and development initiatives that will support clinical decision making across a variety of disease areas. Diagnostics has always been an indispensable component of healthcare, and the alignment of both science and technology makes for the perfect fit in an area for high potential impact, especially in pressure-driven environments,” says Dr. Peter Fitzgerald, Managing Director of Randox Laboratories Ltd. Bosch Healthcare Solutions and Randox already collaborated during the Covid-19 pandemic. In spring 2020, Bosch launched one of the world’s first fully automated PCR tests for the SARS-CoV-2 coronavirus. Together with Randox, the rapid test for use in doctor’s offices, nursing homes, testing stations, and hospitals was made ready for the Vivalytic analysis device within the space of just a few weeks.
Development goal: sepsis test based on BioMEMS technology
One focus of the development partnership with Randox is the implementation of a highly sensitive multiplex2test for sepsis on the Vivalytic analysis platform.Sepsis, also known as “blood poisoning,” is a potentially life-threatening complication3 that can occur in conjunction with various infectious diseases. A medical emergency that can lead to multiple organ failure, it requires immediate medical treatment. The planned IVD grade sepsis test is to be based for the first time on the highly innovative and novel BioMEMS technology developed by teams from Bosch corporate research in Renningen and Bosch Healthcare Solutions in Waiblingen. “We have set ourselves the ambitious goal
of adding the functions of a high-performance silicon chip based on microsystems technology to our test cartridges for the Vivalytic platform. In doing so, we will combine Bosch’s unique expertise in the areas of MEMS chips, molecular diagnostics, and microfluidics,” Marc Meier says. “Clinical outcomes in sepsis depend on timely diagnosis and appropriate early therapeutic intervention. Current methods of sepsis diagnosis are insufficient and time-consuming. With over 10 years of experience in the field of infectious diseases diagnostics, we aim to develop a state-of-the-art sepsis test using the highly multiplexing BioMEMS technology. Such a test could revolutionize sepsis diagnosis, ultimately leading to improved treatment outcomes and lower mortality rates from this life-threatening condition,” says Dr. Peter Fitzgerald.
Smaller and faster: from microfluidics to nanofluidics – thanks to BioMEMS
The powerful BioMEMS chip adds a further innovative analysis method to the Vivalytic test cartridge, enabling it to test simultaneously and significantly faster for a large number of different pathogens. It is called BioMEMS because it combines microelectromechanical systems (MEMS) with microfluidics for applications in the field of medical technology. In microfluidics, very small amounts of fluid in the microliter range are moved in a very small space. Miniaturization allows qualitative biochemical polymerase chain reactions (PCR) to run in parallel in real-time on a single BioMEMS chip. “Compared to previous PCR reactions, the volumes of liquids are reduced by a factor of 1,000 to the nanoliter range. The analysis of liquids is left to a small microchip,” Marc Meier explains. With the new BioMEMS technology, fully automatic testing up to 250 genetic characteristics4 (e.g. pathogens) in one cartridge is possible in less
than 15 minutes. The test cartridge is a highly complex laboratory the size of a smartphone, so to speak. Another future advantage of BioMEMS will be simpler and faster adaptation of new tests or existing tests on the chip itself. For example, existing tests can easily be expanded to include additional features. “The BioMEMS technology paves the way for us to move into nanofluidics, where each pathogen will be examined in a reaction vessel the thickness of a hair,” Meier says. To achieve this, Bosch wants to create more capacity on a MEMS chip and expand it what are known as “nanocavities”. Thanks to t
hese very small cavities, even more biochemical processes will be able to run in parallel on a
chip. With increasing miniaturization, the technology has potential to be used in oncology as well over the long term. The BioMEMS chips are to be manufactured at the Bosch semiconductor plant in Reutlingen, with bio-integration and cartridge
assembly to be carried out at Bosch Healthcare Solutions in Waiblingen.
Vivalytic platform: easy application at the point of sample collection
The advantages of carrying out PCR tests on the Bosch Vivalytic platform lie not only in speedy a
nalysis, but also in the ease of use: Once the sample has been taken, it is placed in the test cartridge. The cartridge, which contains all the necessary reagents for the respective test, is then inserted into the Vivalytic analyser for automated evaluation. Medical staff require only brief training on how to operate it. This enables fast and targeted diagnostics directly at the point of sample collection – either at the doctor’s office or in the hospital – without the often long and time-consuming detour via a central laboratory. Bosch Healthcare Solutions already distributes various tests for diseases of the upper and lower respiratory tract, such as SARS-CoV-2 – also a pooling variant and as a saliva test – or a test to differentiate between SARS-CoV-2, RSV virus and influenza. There are also tests for pathogens that cause sexually transmitted infections (STI) and MRSA / SA (“hospital germ”). Starting in summer, it is planned to expand the portfolio with tests for whooping cough (Bordetella holmesii, Bordetella parapertus
sis, and Bordetella bronchiseptica), urinary tract infections (UTI), bacterial meningitis, the two most common sexually transmitted diseases (Chlamydia trachomatis; CT and Neisseria gonorrhoeae; (NG), fungal infections (Candida auris), and three tests for diarrheal diseases
(norovirus, Clostridioides difficile, HSP)
Contact persons for press inquiries:
Robert Bosch GmbH
Dörthe Warnk, Phone: +49 711 811-55508
E-mail: doerthe.warnk@bosch.com
Randox Laboratories Ltd.
Martin Conway, Phone: +44 (0) 28 9442 2413
E-mail: martin.conway@randox.com
Secretary of State visit to Randox Science Park
The Rt Hon Chris Heaton-Harris, Secretary of State for Northern Ireland paid a visit to Randox Science Park on Thursday, October 19th, to discuss Randox capabilities and undertake a tour of the facilities at the Antrim site.
As leading diagnostic company from the UK & Ireland, Randox have spent over forty years improving healthcare, with a focus on the provision of timely and accurate testing both to improve clinical diagnosis and promote preventative healthcare.
The purpose-built facilities at the site, covering research and development, engineering, manufacturing and accredited laboratories provides an unparalleled depth of diagnostic capability with a single site.
More than 5% of the world’s population (over 400 million people) receive medical diagnosis using Randox products each year.
Randox’s proprietary Biochip Technology is the result of a £350 million investment, allowing many tests to be run simultaneously, greatly improving the diagnostic power available to clinicians. This innovative technology allowed the provision of advanced health profiling to support both early diagnosis and the transition to preventative healthcare.
Randox Science Park is a central hub of Randox’s life science manufacturing, engineering and research and development. Randox employ over 2,200 staff, including 800 research scientists and
engineers – all focused on improving life science diagnostic capabilities globally.
Randox Science Park is one of four key manufacturing and development sites, with others located in Dungloe, County Donegal; Bangalore, India; and the Greater Washington DC area, USA. Across the UK & Ireland there is also a growing network of Randox Health Clinics.

Effortless Data Management: Acusera 24.7 Reports
You’ve carried out your daily maintenance and run your IQC. You’ve got your results and now it’s time to type them up into one of your expansive spreadsheets. Reports
You’ve probably got your spreadsheet set up to calculate the required parameters already, but what if there’s an error in the formula? Or what if you make an error when entering your data? Or worse, what if you try to open the spreadsheet only to find that the file is corrupted or lost? If your Excel file is there, someone else might already be editing it, meaning you must wait until they’re finished before you can make any changes.
Even if you face none of these obstacles the labour-intensive statistics needed for performance review and validation might just keep you up at night.
Well, with Acusera 24.7, these concerns are history.
Whether you make use of our automated or semi-automated data entry options, you can be sure that the data put into the system is exactly that returned by your instrument. If you use the manual data entry option, we can’t remove the human error element – but with our simple and intuitive interface, we trust you’ll be flawless anyway. What’s more, the cloud-based nature of our software also means you won’t lose the data by mistake and unique access for each user allows multiple people to be logged in at the same time.

So, what next?
Well, you can view this data on our dashboard for fast and easy access to your results but delve a little deeper into Acusera 24.7 and you can access comprehensive, easy-to-read, customisable, reports designed to speed up the review process.
These reports include statistical analysis, exception reports, peer group statistics, uncertainty of measurement and advanced statistical metrics. The latter two we’ll look at in a dedicated article. For the others, however, let’s dive in and see how you could benefit from our range of extensive reports.
Statistical Analysis Reports
The first report we will look at is the statistical analysis report. This report allows you to view your IQC data from a specified date range, and compare it to your cumulative data, that is, all the IQC data you’ve collected since you began using that lot, as well as the peer group data for the same lot all within one screen. If you are part of a chain of laboratories, you can compare this data with your laboratory group to see how your lab stacks up by using the World/Group toggle button.
This report provides you with the count, mean, SD, CV, SDI and CVI for a lot and can be organised by assay, as shown in the image below, instrument, or method, allowing you full freedom to customise this report to suit your needs. Don’t forget, like all our reports and charts, this data is fully exportable to PDF or Excel for filing or data review.
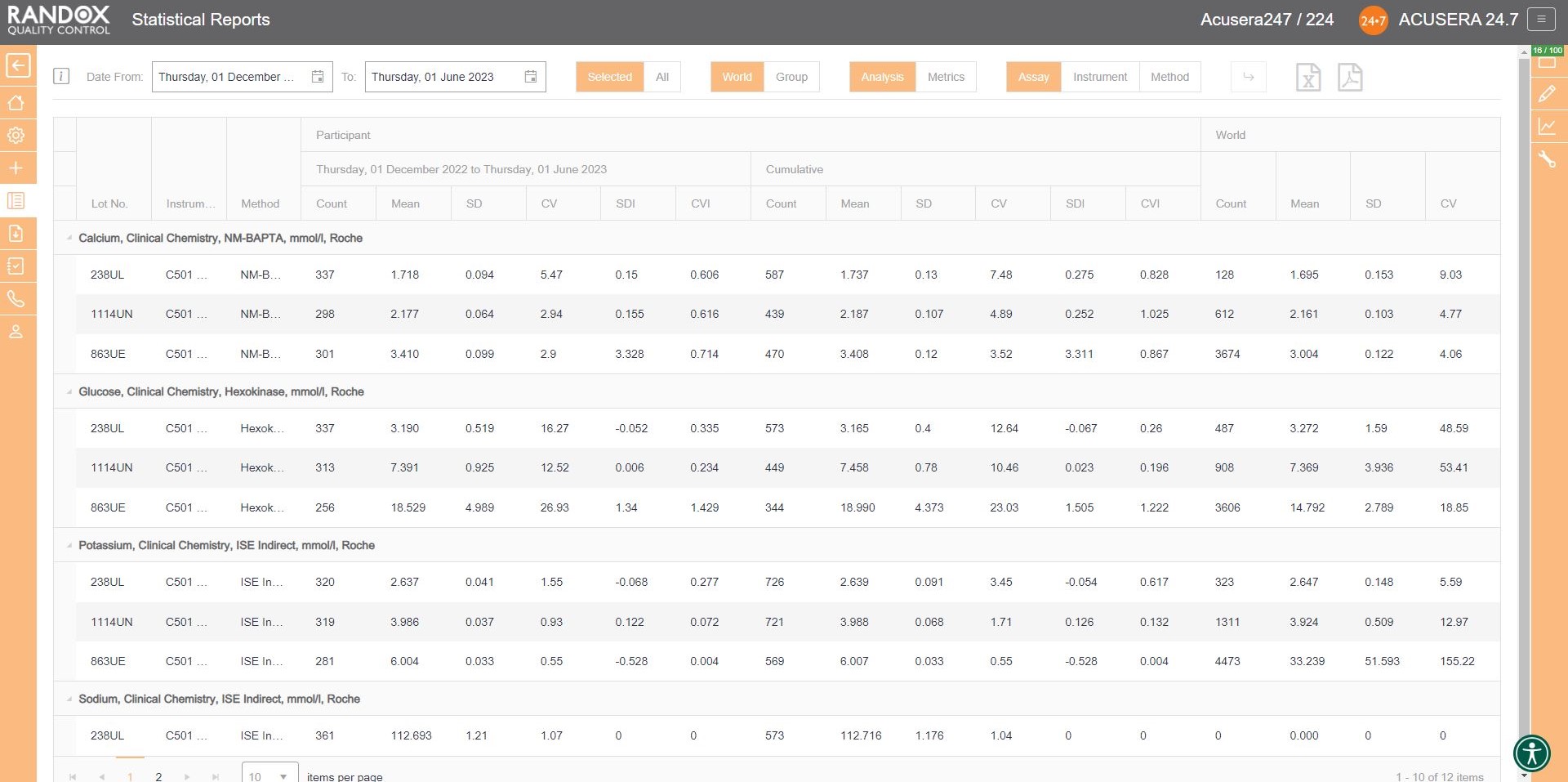
Handy, right? This report provides you with everything you need to carry out the validation and verification of new IQC lots, plus much more. We’ll look at this in more detail in an upcoming article.
Exception Reports
If you wish to determine your best and worst-performing tests, our exception report is perfect for you. This report is designed to quickly and easily identify assays with a high percentage of errors. The exception report provides an on-screen summary of the number of QC results for each individual assay and control lot that fall within the following categories: <2SD, 2-3SD and >3SD. This comprehensive performance review can be filtered: by clicking on the top of the ‘>3DSI’ column, this report will display assays in descending order with your worst-performing assays at the top, as shown below.
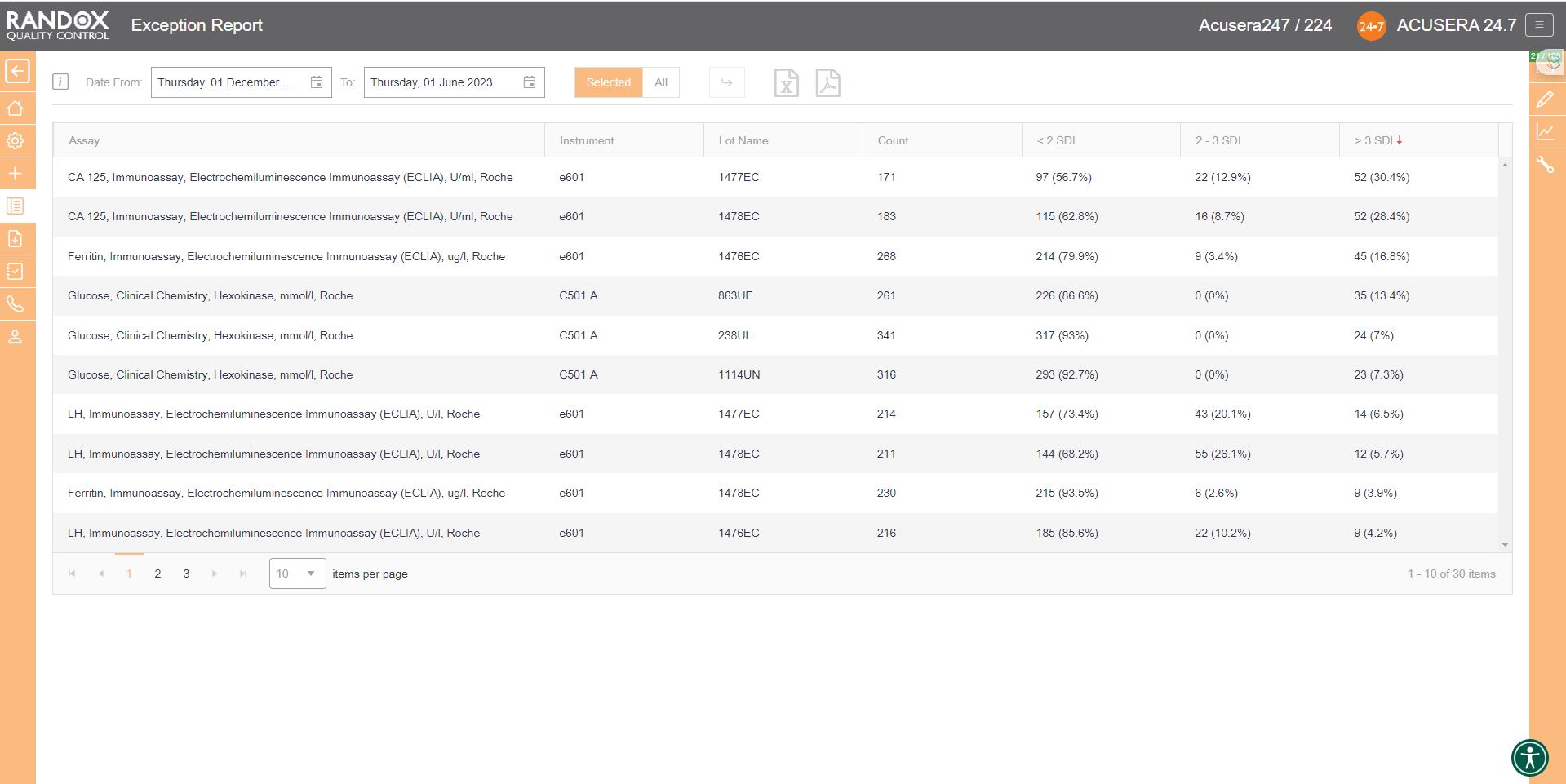
Filtering by ‘<2SDI’, it will display the same data with your best-performing assays at the top.
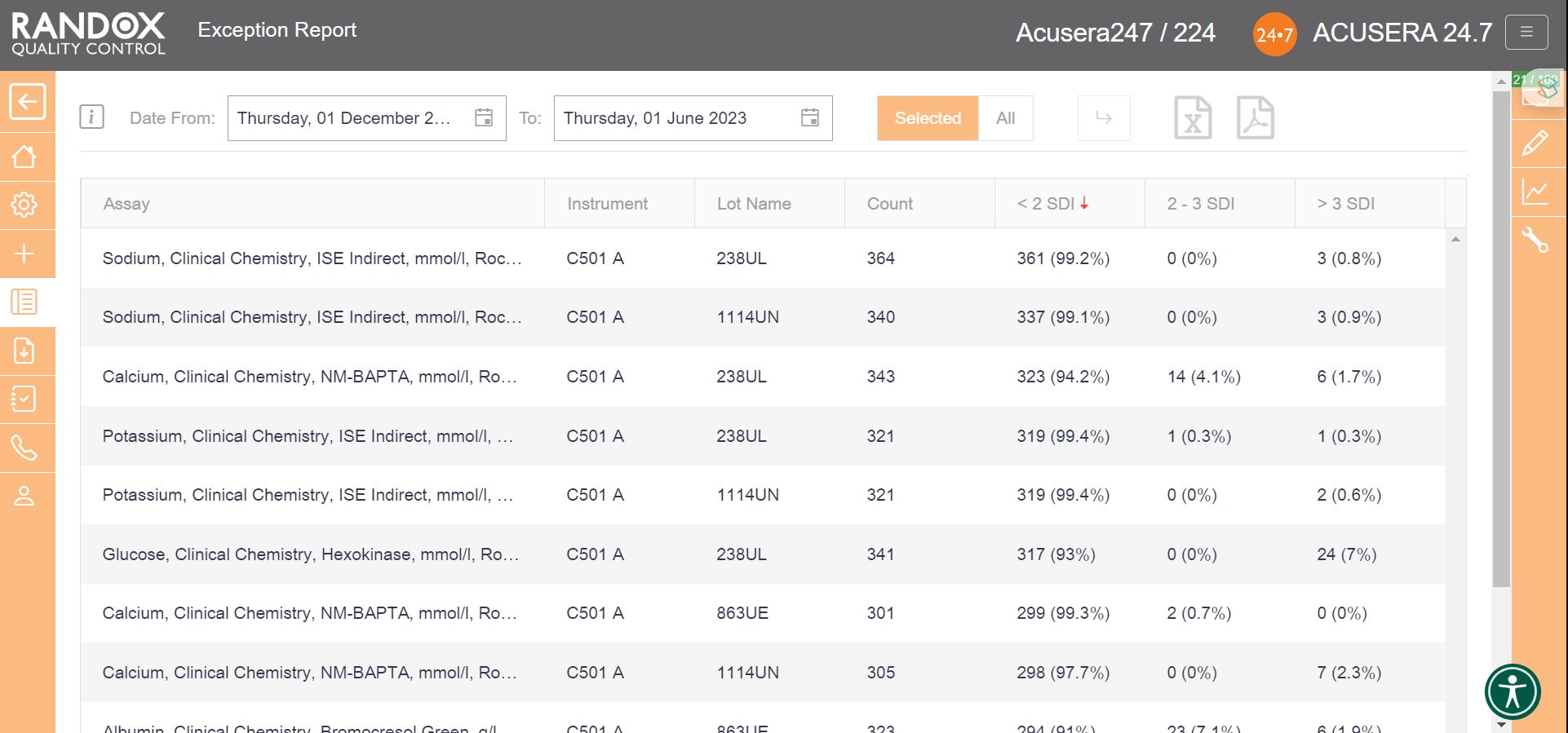
With this information, you can determine in which of your assay’s failures most often occur and encourage staff to look a little more closely at why failures arise and identify changes to improve and minimise errors.
Peer Group Statistics Reports
Now that you have figured out the performance of your assays, you’ll want to see how you compare with others running the same tests. Our Peer Group Statistics Report is your new best friend.
Updated live and in real-time, with no submission deadlines, you can compare your statistics to those of your peer group, determined by analyte, method, instrument manufacturer and model.
Simply select the IQC lot you wish to analyse and Acusera 24.7 will generate the data for you, displaying the count of QC data, mean, SD and CV, giving you comprehensive insight into your performance vs your peers.
You can customise this report even further. If you select an analyte, we’ll show you the data for that analyte alone. If not, we’ll show you the information for all analytes related to that lot. The same goes for specifying a date range – if you choose a range, we’ll show you the data inside that range alone. If not, we’ll show you all the data for your chosen lot.
By clicking on the headers, you can filter the data – 1 click will display the data in ascending order, 2 will show you a list in descending order and 3 clicks will reset the table.
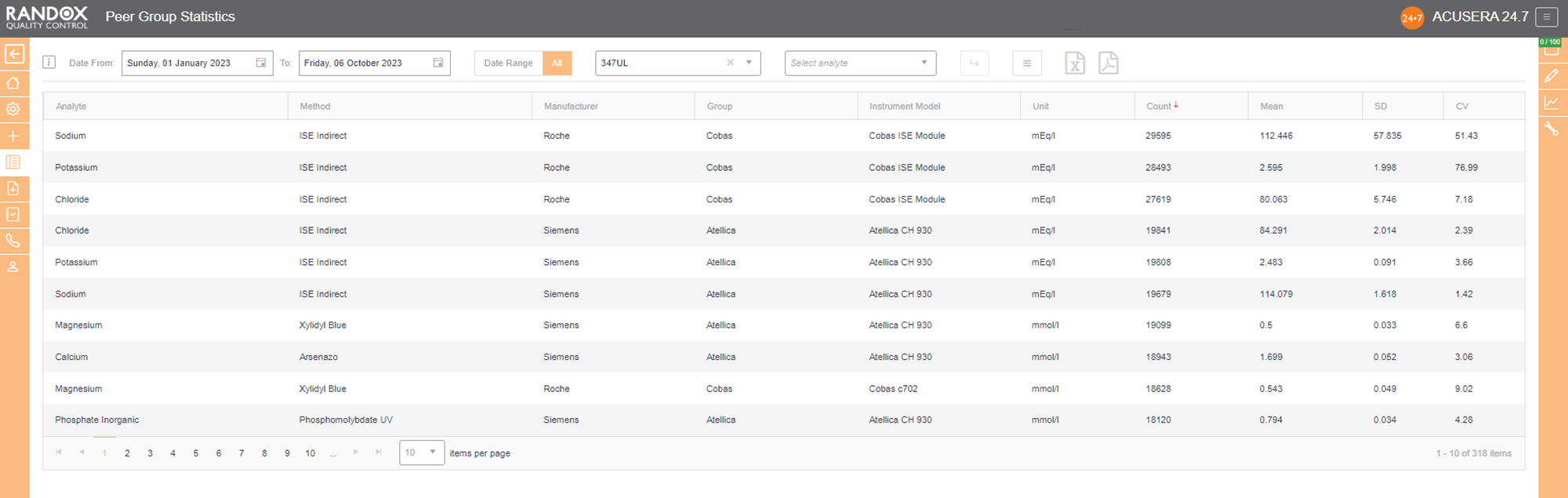
When these reports are combined with the other impressive features of Acusera 24.7, like our fully customisable charts or advanced statistical analysis, this software can help streamline your IQC procedure and data review process.
When the accreditors come knocking, others will be scuffling around trying to gather multitudes of reports and files, but you will be sitting with a smile on your face and your feet up, because you’ve got Acusera 24.7.
With full onboarding assistance and technical support that’s top-of-the-class, you’ll always have someone to help you get to the bottom of any problems that you face.
If you haven’t already booked a demo, get in touch with us today and let us show you how much time we can save you with this innovative and intuitive software. Alternatively, take a look at our Resource Hub for some material on Acusera 24.7 or Acusera IQC.
To streamline your QC Data analysis, get in touch with us at marketing@randox.com.
Acute Kidney Injury and Antimicrobial Stewardship
An estimated 1 in 5 hospital admissions in the UK is associated with acute kidney injury1, providing a clear illustration of the need for novel, rapid detection methods. Our latest whitepaper looks at this common condition and the links between Acute Kidney Injury and Antimicrobial Stewardship. For more details on the things discussed in this article, you can download the full whitepaper below.
Acute Kidney Injury
Acute Kidney Injury is defined as a sudden loss of kidney function. This causes a disruption in the kidneys’ ability to filter waste out of your blood resulting in an accumulation of waste products as well as other imbalances.
The loss of kidney function is the result of a sudden reduction in glomerular filtration rate (GFR), the process through which waste is extracted from the blood and is often reversible2.
Aetiology of Acute Kidney Injury
The differential pressure existing between the glomerulus and Bowman’s is the driving mechanism for glomerular filtration2. This pressure contrast is influenced by the combined resistances of the afferent (leading to the glomerulus) and efferent (leading away from the glomerulus) vascular pathways in the kidney. Under normal kidney function, these resistances are in equilibrium, facilitating the proper functioning of the GFR. For example, an increase in efferent resistance restricts the blood flow out of the kidney, elevating pressure inside the kidney and reducing GFR, and vice versa2. However, in AKI, the decline in renal blood flow and GFR has a pathological origin. The pathophysiology of AKI can be classified as prerenal, intrinsic renal, or postrenal.
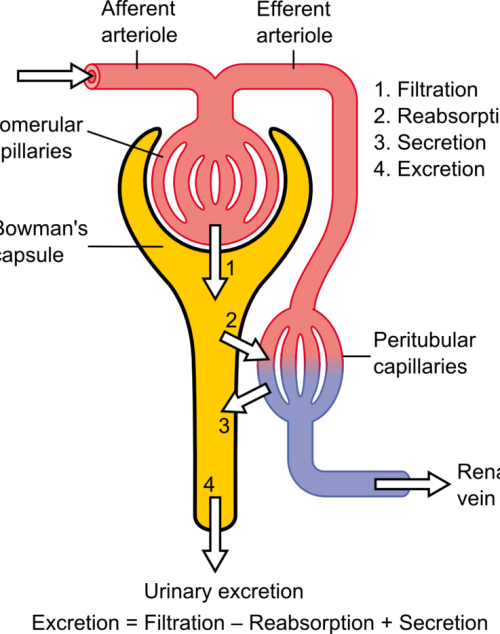
Pre-renal Acute Kidney Injury
Pre-renal AKI is caused by reduced afferent blood flow or, in other words, increased afferent resistance. While tubular and glomerular functions generally remain intact, pre-renal AKI may be caused by systemic hypoperfusion (decreased blood flow) or selective hypoperfusion to the kidney, caused by renal artery stenosis or aortic dissection3.
Intrinsic Renal Acute Kidney Injury
Renal AKI describes the conditions which affect the glomerulus or tubule, for example, acute tubular necrosis and acute interstitial nephritis. This collection of conditions is associated with vasoconstrictor expression in renal afferent pathways2.
Post-renal Acute Kidney Injury
Post-renal AKI usually results from an obstruction in the filtration system. Causes of obstruction include kidney stones, tumours, or blood clots, commonly in the bladder outlet. Obstruction affecting one side might not invariably lead to acute kidney injury, especially when the impediment develops slowly, such as with tumour growth. This is because the unaffected kidney might be able to adjust and make up for the compromised functionality3
Symptoms & Treatment
AKI often manifests with decreased urine output as its primary symptom. However, additional symptoms, when present, can encompass fatigue, nausea, vomiting, or confusion4. To achieve an accurate diagnosis, a comprehensive review of the patient’s medical history and a physical examination are essential to identify the underlying cause of the condition.
The treatment and management of AKI are contingent upon the root cause of the condition. In milder cases, measures are implemented to maintain appropriate levels of fluid, electrolytes, and blood pressure. Nutritional support may also be administered when necessary. In the most severe instances of AKI, dialysis may be warranted to compensate for the diminished kidney function5.
Creatinine serves as a valuable diagnostic tool for evaluating renal conditions, including kidney health, GFR, and muscular dystrophy. However, abnormal serum creatinine (SCr) levels only become evident when a significant portion of the renal mass is compromised. The kidneys possess an impressive capacity to adapt to reduced function, which means that a considerable loss of function or GFR is necessary to influence SCr levels. This poses a challenge when it comes to early detection of AKI6.
Novel biomarkers, KIM-1, NGAL, Clusterin, and Cystatin C, are associated with AKI2 and can be analysed through molecular testing. These new methods can provide a fast and accurate assessment of an individual’s kidney health, at a much earlier stage than SCr quantification2.
Antimicrobial Stewardship
Antimicrobial Stewardship (AMS) programs are specifically crafted to enhance the efficiency of antimicrobial utilization, curtail the emergence of Antimicrobiasl Resistance (AMR), and enhance patient outcomes7. These programs encompass a variety of approaches, such as educational initiatives, training, the establishment of guidelines and protocols, ongoing monitoring and feedback regarding antimicrobial usage, and the management of antimicrobial formularies. Through the promotion of prudent antibiotic utilization, AMS programs contribute to the safeguarding of the efficacy of currently available antimicrobial agents and the deceleration of AMR development7.
Antibiotics and Acute Kidney Injury
Various antibiotics are associated with the progression of AKI due to their nephrotoxicity which can cause severe damage to the kidneys. These antibiotics include polymyxins, aminoglycosides and the commonly used, vancomycin8.

Randox Renal Injury Detection
Using the patented Biochip Technology, the Randox Acute Kidney Injury (AKI) array, available on the Evidence Investigator, simultaneously tests for four novel biomarkers (KIM-1, NGAL, Clusterin, Cystatin C) delivering an early diagnosis and monitoring of treatment efficacy. Multiplex testing better captures reduced renal function, as each biomarker reflects different mechanisms that result in similar injury outputs, allowing for a more accurate picture of the underlying cause of AKI. Along with being able to identify AKI at a much earlier stage, this array provides an accurate and sensitive solution for the diagnosis and monitoring of AKI.
If you’d like some more information on the Randox Acute Kidney Injury Array or would like to add this technology to your laboratory, take a look at our website at https://www.randox.com/acute-kidney-injury/ or get in touch today at marketing@randox.com.

References
- NICE. How common is it? Acute Kidney Injury . Published July 2023. Accessed October 2, 2023. https://cks.nice.org.uk/topics/acute-kidney-injury/background-information/prevalence/
- Adiyanti SS, Loho T. Acute Kidney Injury (AKI) Biomarker.; 2012.
- Manzoor H, Bhatt H. Prerenal Kidney Failure.; 2023.
- NHS. Acute Kidney Injury. NHS. Published 2023. Accessed July 31, 2023. https://www.nhs.uk/conditions/acute-kidney-injury/
- Goyal A, Daneshpajouhnejad P, Hashmi M, Bashir K. Acute Kidney Injury . In: StatPearls [Internet]. StatPearls Publishing ; 2023.
- Rule AD, Lieske JC. The estimated glomerular filtration rate as a test for chronic kidney disease: Problems and solutions. Cleve Clin J Med. 2011;78(3):186-188. doi:10.3949/ccjm.78a.11004
- Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990-1001. doi:10.1016/S1473-3099(17)30325-0
- Clifford KM, Selby AR, Reveles KR, et al. The Risk and Clinical Implications of Antibiotic-Associated Acute Kidney Injury: A Review of the Clinical Data for Agents with Signals from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) Database. Antibiotics. 2022;11(10):1367. doi:10.3390/antibiotics11101367








