Troponin T Quality Control
Troponin T Quality Control

Intended for use with the Roche system, this control is manufactured using only the highest quality material and offers an unrivalled 7-day thawed stability at +2ºC to +8ºC.
Features & Benefits
- Liquid frozen for enhanced stability
- Aqueous based material
- Ultra low levels of Troponin T
- Stable to expiry date at -18-24ºC storage
- Thawed stability of 7 days at 2°C – 8°C
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Troponin T Control | 6 x 3 ml | 1 | CQ10450 | |
Analytes
- Ultra low Troponin T
Ethanol Calibrator/Control Set

Dedicated calibrator and control set designed for the calibration and quality control of the Randox Ethanol assay.
Features & Benefits
- Liquid ready-to-use
- Human urine
- Stable to expiry date when capped and stored at 2oC – 8oC
- Open vial stability of 28 days at 2oC – 8oC
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Ethanol Calibrator/Control Set | 4 x 10ml | 1 | DA2703 | |
Analytes
Ethanol
Related Products
sTfR Quality Control

Providing a true third party solution for the measurement of Soluble Transferrin Receptor (sTfR), the Acusera sTfR Control will deliver an unbiased, independent assessment of analytical performance.
Designed for use with sTfR assays, this handy single analyte control saves money on wasted material.
Features & Benefits
- Lyophilised control
- Human based material
- Assayed target values available
- Stable to expiry date at 2°C to 8°C
- Reconstituted stability of 30 days at 2°C to 8°C
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| sTfR Control (Bi-level) | 3 x 2 x 1 ml | 1 | TF10162 | |
| sTfR Calibrator | 6 x 1 ml | 1 | TF10161 | |
Analytes
- Soluble Transferrin Receptor (sTfR)
Related Products
Metabolic Syndrome Array II Quality Control

A multi-analyte quality control with target values and ranges provided for 3 parameters associated with metabolic syndrome.
Features & Benefits
- Lyophilised for enhanced stability
- 100% human material
- Stable to expiry date at 2oC – 8oC
- Reconstituted stability of 72 hours at 2oC – 8oC and 7 days at -20°C
- Assayed values available for Randox Biochip systems
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Metabolic Syndrome Array II Control | 3 x 3 x 1ml | 3 | EV3761 | |
| Metabolic Syndrome Array II Calibrator | 9 x 1 ml | 3 | EV3760 |
Analytes
- Adiponectin
- CRP
- Cystatin C
RX series (Concept 3)

The RX series range of clinical chemistry analysers includes both semi-automated and fully automated testing for a range of clinical settings. With a world leading test menu comprising of routine chemistries, specific proteins, lipids, therapeutic drugs, drugs of abuse, antioxidants and diabetes testing, the RX series offers laboratories the complete clinical chemistry package and results you can trust. The RX series was built with three core values in mind – Reliability, Accuracy and Precision.

Consolidation of Routine & Specialised Testing on One Single Platform
With an extensive product portfolio covering over 100 disease markers within routine and nice testing, the RX series removes the need for a separate nephelometry system for specific proteins and allows laboratories to bring all testing in-house; thus ensuring minimal downtime and providing real cost savings through consolidation.
![]()
Low Reagents & Sample Volumes
Built with excellence in mind, the RX series range of analsyers require a low sample volume to deliver consistent high quality results which is beneficial when working with paediatric patients and animals. Combined with our high quality reagents, the RX series reduce the possibility of misdiagnoses, offering accurate, reliable and precise results each time, every time.

Unrivalled Customer Support
Our team of trained engineers are on hand to work with you in preserving the continuity of your operations while maximising the potential of your RX series instrument. We know time is critical in any laboratory and our global network means we are uniquely positioned to meet your needs with local service and support whenever you need it.
Fully Automated
Test Menu
Semi-Automated
Test Menu
Niche
Test Menu
QCMD – Molecular External Quality Control
QCMD is a world leading External Quality Assessment (EQA) / Proficiency Testing (PT) scheme, dedicated to improving the quality of molecular diagnostic assays used in the detection of infectious diseases.
With an extensive database of over 15,000 participants in over 120 countries, QCMD is one of the largest providers of molecular EQA in the field of molecular diagnostics.
Blood Borne Viruses
Central Nervous System Diseases
Drug Resistance
Exotic / Emerging Diseases
Gastrointestinal Diseases
Immunocompromised Associated Diseases
Multiple Pathogen / Syndromic Infections
Respiratory Diseases
Serology
Sexually Transmitted Infections
Transplant Associated diseases
Typing
New Pilot Studies

After the close of the results return phase, EQA participants will receive an individual report outlining their performance relative to their method and technology groups. A supplementary report may be commissioned – this includes any additional relevant information regarding the annual EQA distribution, as well as scientific expert commentary and feedback on the overall results within that distribution.
*Randox are authorised by QCMD to provide the QCMD EQA schemes under a strategic global partnership. The EQA design, composition, data analysis & reporting remain the responsibility of QCMD. Please refer to specific geographical regions for further details on availability.
Explore Further
Contact Us
QCMD Website
RIQAS EQA
Protected: RX series (Concept)
The Benefits of True Third Party Controls
In the clinical laboratory, Quality Control (QC) refers to the process of detecting analytical errors to ensure both the reliability and accuracy of patient test results. Poor performance can result in misdiagnosis, delayed/inappropriate treatment, increased costs and may even be potentially life threatening for the patient.
Dependent / First Party Controls
Dependent controls refer to any control material that has been produced by the instrument or reagent manufacturer for use on a specific test system. Such controls are often manufactured from the same raw materials as the calibrator, making them less sensitive to subtle changes in performance.
As dependent controls are generally optimised for use with the manufacturer’s test system, these controls can mask weaknesses, and therefore, are increasingly considered less effective than independent controls.
Semi-Dependent Controls
Semi-dependent control material, although produced independently of the instrument or reagent, is often supplied or recommended by the instrument/reagent manufacturer. It is this manufacturing relationship between the two that requires close scrutiny when considering if these controls are fit-for-purpose.
Although the control material is not produced by the instrument manufacturer, it is produced according to their exact specifications and therefore, optimised to work with a specific platform.
Independent / Third Party Controls
Independent or third party quality control material has not been designed or optimised for use with any instrument, kit or method. This complete independence enables the quality control material to closely mirror the performance of patient samples, and in doing so, provide an unbiased, independent assessment of analytical performance across multiple platforms.

Third party controls have been designed to deliver an independent, unbiased assessment of performance with any instrument or method helping you gain accreditation. Below are some of the key benefits of third party controls:
- Values assigned using a large number of independent laboratories ensuring statistically valid targets.
- Highly consolidated controls allow for space, time, and ultimately, cost savings.
- Boosted shelf life ensures continuity of supply and reduced costs
- Reduced preparation times by removing the need for multiple instrument controls
Regulatory Requirements
Third party controls are growing in popularity across the globe. More and more laboratories are beginning to use third party controls as part of their daily QC strategy. The benefits of such controls are widely accepted and recommended by both key opinion leaders and regulatory bodies in the field of Quality Control.
“Use of independent third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer”
ISO 15189:2012 Section 5.6.2.2
ISO 15189:2012 Medical Laboratories – Requirements for Quality and Competence
The benefit of running third party controls in your laboratory cannot be underestimated. The following case studies highlight their many benefits and how they have helped laboratories across the world to provide more accurate and reliable test results.
Case Study One: Identifying Lot-to-Lot Variability with Third Party Controls
A laboratory in the UK contacted Randox Technical Services, reporting higher than expected QC results for
Thyroglobulin. When using a third party control (Acusera Immunoassay Premium Plus) the results were four
times higher on their main analyser compared to other systems. However, when they ran the instrument
manufacturer’s control alongside the third party control it did not show the same problem.
After reviewing EQA data, the Technical Services team confirmed there was a significant difference in results
compared to other instruments, and set about helping the laboratory troubleshoot. After an exhaustive
review of procedures and processes, the customer contacted the instrument manufacturer, who advised of a
positive bias with several batches of reagent, including the batch the laboratory was using.
Conclusion: By using a third party control the laboratory was able to detect a shift in results after changing
reagent batch that the instrument manufacturer’s control did not.
Case Study Two: Overcoming Instrument Errors with Third Party Controls
A laboratory using the Acusera Assayed Chemistry Premium Plus control contacted Randox Technical Services
after observing a consistent negative bias for ALT which was not replicated by the instrument control. They
had previously contacted their instrument manufacturer who advised that the problem was with the control
and not the reagent or instrument.
Randox investigated the problem and demonstrated that patient results were also wrongly reported low. This
later led the instrument manufacturer to recommend a wash stage to eliminate any interference.
Conclusion: The use of a third party control in this instance enabled the identification of a procedural error
with the instrument that the recommended control did not.
Explore the benefits of Randox Acusera third party controls below.
Our extensive range of assayed quality controls are supplied with highly accurate target values for a wide range of instruments and methods. Our unique value assignment process utilises thousands of independent laboratories globally ensuring target values won’t change throughout the shelf life of the control and eliminates the need to spend time and money performing value assignment in-house.
Accuracy coupled with unrivalled traceability to International Reference Laboratories, provides a product of unsurpassed accuracy and reliability.
We take quality seriously, that’s why all QC products are manufactured to the highest possible standard delivering control products of unrivalled quality. Our superior manufacturing processes ensure stability claims and analyte levels won’t differ significantly from lot to lot. You can therefore be sure of receiving the same standard of product time and time again.
Regular shifts in QC results when a reagent batch is changed can be both costly and frustrating for many labs, resulting in a frequent need to reassign target values. Designed to be commutable, the Acusera range of Internal Quality Controls will react to the test system in a manner as close as possible to the patient sample helping you to meet ISO 15189:2012 requirements while ultimately ensuring accurate & reliable instrument performance. Furthermore our lyophilized controls contain no added preservatives or stabilisers ensuring a sample matrix that closely matches the patient sample.
ISO 15189:2012 states, “The laboratory shall use quality control materials that react to the examining system in a manner as close as possible to patient samples”.
All controls for use with immunoassay/immunology based methods are manufactured using only 100% human components demonstrating our commitment to quality and eliminating costly QC shifts when reagent batch is changed.
Working stability and product shelf life are important considerations for any lab when choosing which internal quality control material is best suited to their needs. Labs often spend up to one month validating new material, a process which can be minimised by opting for a control with an extended shelf life. At Randox our lyophilised controls have a shelf life of up to four years and our liquid controls a shelf life of up to two years from the date of manufacture ensuring continuity of lot supply and ultimately reducing the need for expensive new lot validation studies.
Each of our third party controls will have its own reconstituted or open vial stability, some of the analytes will have limitations, however we pride ourselves on not misleading customers with false claims. The extended open vial and reconstituted stabilities will help laboratories to minimise waste and reduce costs.
Randox is a leading provider of multi-analyte, third party controls designed to allow any lab to carry out highly accurate QC using fewer controls. In an industry where budgets and resources are increasingly under pressure, highly consolidated controls will ensure high levels of throughput without compromising on accuracy. Uniquely comprising up to 100 analytes in a single control product, costs, preparation time and storage space are dramatically reduced without sacrificing on quality.
The presence of analytes at key decision levels in all Acusera controls will not only ensure accurate test system performance across the clinical range, but will further aid consolidation and maximise laboratory efficiency by eliminating the need to purchase additional high or low level controls, which are often expensive.
Available in multiple levels, the Acusera range of third party controls are designed to challenge laboratory instruments throughout the patient reportable range. The presence of analytes at clinically relevant decision levels not only helps to ensure accurate instrument performance but maximizes laboratory efficiency by eliminating the need to purchase additional low/high concentration controls at extra expense.
Randox also employs easy to use colour-coded packaging to help distinguish between different levels and reduce costly mix-ups.
ISO 15189:2012 states, “The laboratory should choose concentrations of control materials wherever possible, especially at or near clinical decision values, which ensure the validity of decisions made”.
Randox Acusera is a world leading manufacturer of true third party controls providing a cost effective, high quality solution for any lab – regardless of their size or budget. Designed to provide an unbiased, independent assessment of performance, our internal quality controls have not been manufactured in line with, or optimised for use with any particular reagent, method or instrument helping you to easily meet ISO 15189:2012 recommendations.
ISO 15189:2012 states that the “use of independent third party control material should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer.”
Traceability refers to the property of a measured result or calibrator value to be related or traced back to a reference measurement procedure or reference material through a series of measurements with known uncertainties.
The European parliament and council directive 98/79 EC require values assigned to both calibrators and control materials on in vitro diagnostic medical devices to be traceable to a recognised reference material or reference measurement procedure of higher order, e.g. SI units.
Guidelines have been set for diagnostic manufacturers to follow when assigning calibrator values and establishing traceability. These guidelines were set by the European standards EN/ISO 17511 and also EN/ISO 18153. The Randox traceability pathway has been established with reference to standards ISO 17511 and ISO 18153. The pathway has been followed to establish traceability for Randox calibrators allowing all patient results to be traced right back to the source.
With an extensive range of assayed/unassayed, liquid/lyophilised and single/multi-analyte controls, the Acusera portfolio has a solution to suit all laboratory preferences.
Custom Controls
Randox is a market leader in the manufacture of customised quality controls designed to meet the individual and unique requirements of even the most specialised laboratories.
Acusera Third Party Controls
Download Third Party Benefits Brochure
Contact Us
Acusera Internal Quality Control Analyte List
Quality Control is our passion; we believe in producing high quality material that can help streamline procedures, whilst saving time and money for laboratories of all sizes and budgets. With an extensive product offering comprising third party controls and calibrators, interlaboratory data management, external quality assessment, and calibration verification, you can count on Randox to deliver trustworthy results time and time again. Just ask one of our 60,000 users worldwide.
Our Acusera Internal Quality Control A – Z analyte list highlights how comprehensive our Acusera product portfolio is. Search through the list to see if we have the analyte you require.
Acusera Parameter List
#
5-HIAA
17-OH-progesterone
17β Clostebol
1-25-(OH₂)-Vitamin D
25-OH-Vitamin D
A
α-1-Acid Glycoprotein
α-1-Antitrypsin
α-1-Globulin (Electrophoresis)
α-2-Globulin (Electrophoresis)
α-2-Macroglobulin
α-Fetoprotein (AFP)
α-HBDH
ACE (Angiotensin Converting Enzyme)
Acetaminophen Acid Phosphatase (Non-Prostatic)
Acid Phosphatase (Prostatic)
Acid Phosphatase (Total)
ACTH
Active Vitamin B12 (Holotranscobalamin/HoloTC)
Activated Partical Thromboplastin Time(APTT)
AHD Albumin
Albumin (Electrophoresis)
Aldolase x Aldosterone
Alkaline Phosphatase (ALP)
ALT (GPT)
AMH
Amikacin
Ammonia
AMOZ Amylase
Amylase (Pancreatic)
Androstenedione
Anti-HAV
Anti-HBc
Anti-HBe
Anti-HBs
Anti-HCV
Anti-HIV 1 / 2
Anti-HTLV 1 / 2
Anti-Streptolysin (ASO)
Anti-Thyroglobulin (Anti-TG)
Anti-Thyroperoxidase (Anti-TPO)
Anti-Thrombin III (AT III)
AOZ Apolipoprotein A-I
Apolipoprotein A-II
Apolipoprotein B
Apolipoprotein C-II
Apolipoprotein C-III
Apolipoprotein E
AST (GOT)
β-Globulin (Electrophoresis)
β-2-Microglobulin
BASO-X
BASO-Y
Basophils (BASO)
Basophils % (% BASO)
Bicarbonate
Bile Acids
Bilirubin (Direct)
Bilirubin (Total)
Blood Bone Alkaline Phosphatase (B-ALP)
Borrelia burgdorferi IgG
Borrelia burgdorferi IgM
Brain Natriuretic Peptide (BNP)
C-Peptide
C-Telopeptide
CA 15-3
CA 19-9
CA 72-4
CA 125
Caffeine
Calcitonin
Calcium
Carbamazepine
CEA
Ceftiofur
Ceruloplasmin
Chloramphenicol
Chloride
Cholesterol (HDL)
Cholesterol (LDL)
Cholesterol (Total)
Cholinesterase
CK-MB
CK (Total)
Complement C3
Complement C4
Copper
Cortisol
CRP
Creatinine
Cyclosporine
Cytomegalovirus (CMV) IgG
Cytomegalovirus (CMV) IgM
CYFRA 21
Cystatin C
D-3-Hydroxybutyrate
D-dimer
Deoxypyridinoline
DHEA-Sulphate
DIFF-X
DIFF-Y
Digoxin
Dopamine
E-Selectin (E-SEL)
Eosinophils (EOS)
% Eosinophils (% EOS)
Epidermal Growth Factor (EGF)
Epinephrine
Epstein Barr Virus (EBV) EBNA IgG
Epstein Barr Virus (EBV) IgM
Epstein Barr Virus (EBV) VCA IgG
Estriol
Ethanol
Ethinylestradiol
Ethosuximide
Factor II
Factor V
Factor VII
Factor VIII
Factor IX
Factor X
Factor XI
Factor XII
Ferritin
Fibrinogen
Folate
Fructosamine
FSC-X
FSH
G-6-PDH
γ-Globulin (Electrophoresis)
γGT
Gastrin
Gentamicin
Gestagens (Generic)
GLDH
Glucose
Glutamate
Glutathione Peroxidase (Ransel)
Glutathione Reductase
Glycerol
GM-CSF
Growth Hormone (GH)
Haematocrit (HCT)
Haemoglobin (HGB)
Haemoglobin (Total)
Haemolysis (H)
Haemopioetic Progenitor Cell (HPC)
Haptoglobin
HAV IgM
HbA1c
HBc IgM
HBeAg
HBsAg
hCG
Free β-hCG
Total β-hCG
HDL-3
Helicobacter pylori IgG
Herpes Simplex Virus 1 (HSV-1) IgG
Herpes Simplex Virus 1 (HSV-1) IgM
Herpes Simplex Virus 2 (HSV-2) IgG
Herpes Simplex Virus 2 (HSV-2) IgM
HIV-1 P24Ag
Homocysteine
hsCRP
Icterus (I)
IMIDC
IMIRF
Immature Granulocytes (IG)
% Immature Granulocytes (% IG)
Immature Myeloid Information (IMI)
Immature Platelet Fraction (IPF)
Immunoglobulin A (IgA)
High Sensitivity Immunoglobulin A (hsIgA)
Immunoglobulin E (IgE)
Immunoglobulin G (IgG)
High Sensitivity Immunoglobulin G (hsIgG)
Immunoglobulin M (IgM)
High Sensitivity Immunoglobulin M (hsIgM)
Inhibin A
Insulin
Insulin Like Growth Factor (IGF 1) x
Intercellular Adhesion Molecule-I (ICAM-I)
Interferon-γ (IFN-γ)
Interleukin-Ia (IL-la)
Interleukin-1β (IL-1β)
Interleukin-2 (IL-2)
Interleukin-4 (IL-4)
Interleukin-5 (IL-5)
Interleukin-6 (IL-6)
Interleukin-8 (IL-8)
Interleukin-10 (IL-10)
Interleukin-15 (IL-15)
Iron
Iron (TIBC)
Iron (UIBC)
Kappa Light Chain
Ketones
L-Selectin (L-SEL)
Lactate
Lactate Dehydrogenase (LDH)
Lambda Light Chain
Lambda Light Chain (Free)
LAP
Leptin
Leukocytes
Lipase
Lipemia (L)
Lipoprotein (a)
Lithium
Luteinising Hormone (LH)
Lymphocytes (LYMPH)
% Lymphocytes (% LYMPH)
Magnesium
Matrix Metalloproteinase-9 (MMP-9)
Measles IgG
Mean Corpuscular Haemoglobin (MCH)
Mean Corpuscular Haemoglobin Concentration (MCHC)
Mean Corpuscular Volume (MCV)
Mean Platelet Volume (MPV)
Metanephrine
Methandriol
Methotrexate
Methyltestosterone
Microalbumin
Macrophage Inflammatory Protein-1a(MIP-1a)
Monocytes (MONO)
Monocytes % (% MONO)
Monocyte Chemoattractant Protein-1 (MCP-1)
Mumps IgG
Myoglobin
N-MID Osteocalcin (OC)
N-Telopeptide
NEFA
Neuron-Specific Enolase (NSE)
Neutrophils (NEUT)
Neutrophils % (% NEUT)
Neutrophil Gelatinase-associated Lipocalin (NGAL)
Nitrite
Norepinephrine
Normetanephrine
NT-proBNP
Nucleated Red Blood Cells (NRBC)
Nucleated Red Blood Cells % (% NRBC)
Nucleated Red Blood Cells X (NRBC-X)
Nucleated Red Blood Cells Y (NRBC-Y)
Oestradiol
Osmolality
Osteocalcin
Oxalate
Oxyhaemoglobin
P-Selectin (P-SEL)
Paracetamol
PAPP-A
pCO2
pH
Phenobarbital
Phenobarbitone
Phenytoin
Phosphate (Inorganic)
PIGF
Plasminogen
Plasminogen Activator Inhibitor
Platelet Distribution Width (PDW)
Platelet Large Cell Ratio (P-LCR)
Plateletcrit (PCT)
Platelet (PLT)
Platelet Optical Count (PLT-O)
pO2
Potassium
Prealbumin
Primidone
Procalcitonin
Procollagen Type 1 N-Terminal Propeptide (P1NP)
Progesterone
Prolactin
Protein C
Protein S
Protein (Total)
Prothrombin Time (PT)
Pyridinium Crosslinks
Pyridinoline
PSA (Free)
PSA (Total)
PTH (Parathyroid Hormone)
PTH (Intact)
Quinolones (Generic)
Red Blood Cell Y (RBC-Y) x
Red Blood Cell Distribution Width CV (RDW-CV) x
Red Blood Cell Distribution Width SD (RDW-SD) x
Renin
Resistin
Retinol Binding Protein (RBP)
Rheumatoid Factor (RF)
Rubella IgG
Rubella IgM
Salicylate
Semicarbazine (SEM)
Sex Hormone Binding Globulin (SHBG)
sFlt-1
sLDL
Sodium
Soluble IL-2 Receptor a (sIL-2Ra)
Soluble IL-6 Receptor (sIL-6R)
Soluble Transferrin Receptor (sTfR)
Soluble Tumour Necrosis Factor Receptor 1 (sTNFR I)
Soluble Tumour Necrosis Factor Receptor 11 (sTNFR I1)
Specific Gravity
Streptomycin
Superoxide Dismutase (Ransod)
T Uptake
T3 (Free)
T4 (Free)
T3 (Total)
T4 (Total)
Testosterone
Testosterone (Free)
Tetracyclines (Generic)
Theophylline
Thiamphenicol
Thrombin Time (TT)
Thyroglobulin
Tobramycin
Total Antioxidant Status (TAS)
Toxoplasma gondii IgG
Toxoplasma gondii IgM
Transferrin
Treponema pallidum (Syphilis) IgG
Triglycerides
Trimethoprim
Troponin I
Troponin T
TSH
Tumour Necrosis Factor a (TNFa)
Tylosin
Unconjugated Oestriol
Urea
Uric Acid (Urate)
Urine Osmolality
Urobilinogen
Valproic Acid
Vancomycin
Vanillylmandelic Acid (VMA)
Varicella Zoster Virus (VZV) IgG
Vascular Cell Adhesion Molecule-1 (VCAM-1)
Vascular Endothelial Growth Factor (VEGF)
Vitamin B12
White Blood Cells (WBC)
White Blood Cells Differential (WBC-D)
Zinc
What is Measurement of Uncertainty?
Measurement Uncertainty (MU) relates to the margin of doubt that exists for the result of any measurement, as well as how significant the doubt is. For example, a piece of string may measure 20 cm plus or minus 1 cm, at the 95% confidence level. As a result, this could be written: 20 cm ±1 cm, with a confidence of 95%. Therefore, we are 95% sure that the piece of string is between 19 cm and 21 cm long.
Standards such as ISO 15189 require that the laboratory must determine uncertainty for each test. However, they have not specified how this should be done.
How do we calculate Measurement Uncertainty using QC data?
Employing your QC data to calculate uncertainty makes several assumptions; your test system is under control, the patient samples are treated in the same manner as your controls and gross outliers have been removed. If you choose to use your QC data to calculate this you should ensure that you use a commutable control with a matrix similar to that of a patient sample, with analytes present at clinically relevant levels
To calculate MU, labs must look at the intra-assay precision and inter-assay precision of their test.
Intra-assay precision: Sometimes known as ‘within run’ precision, is where 20 or more replicates of the same sample are run at the same time, under the same conditions (calculated from a single experiment). Intra-assay precision helps to assess systematic uncertainties
Inter-assay precision: Sometimes known as ‘between run’ precision, is where 20 or more replicates are run at different times – e.g. 1 replicate every day for 20 days (can be calculated from routine IQC data). Inter-assay precision can help identify random uncertainties within the test system.
*The Australian Association of Clinical Biochemists (AACB) recommends that at least 6 months’ worth of QC data are used when calculating the inter-assay precision1.
Once the data is collected, you must calculate the standard error of the mean (SEM) of the intra-assay precision (A) and the SD of the inter-assay precision (B) in order to measure the uncertainty (u). Once A and B have been calculated, they need to be squared, added together and the square root of the sum found:
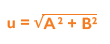
As uncertainty is calculated as SD and 1SD is equal to 68% confidence on a standard Gaussian curve, we can conclude that if we multiply using a coverage factor of 2, we can attain 2SD confidence of 95%. This is known as the Expanded Uncertainty (U):
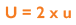
What is the Advantage of Measurement Uncertainty for a lab?
Labs need to carry out MU as it is a requirement of ISO 15189. It states: “The laboratory shall determine measurement uncertainty for each measurement procedure, in the examination phases used to report measured quantity values on patients’ samples. The laboratory shall define the performance requirements for the measurement uncertainty of each measurement procedure and regularly review estimates of measurement uncertainty”.
MU also helps determine whether the difference between two results is negligible due to uncertainty or significant due to a genuine change in condition of the patient; giving labs a greater confidence in reported results.
How can Randox help?
Our new Acusera 24.7 Live Online software provides automatic calculation of MU, saving valuable time and helping labs meet ISO 15189 requirements with ease.
Contact marketing@randox.com to find out how your lab can benefit from Acusera 24.7 Live Online












