Vivalytic | Customer Support

Vivalytic | Customer Support
–
Molecular Diagnostics at the Point of Care
Video Tutorials
For your support, we have compiled a series of informative video tutorials on how to install and operate the Vivalytic machine. You can use these guides to refresh your knowledge from prior training or to learn about specific procedures step by step.
Installation and Operational Guide
Instructional Promo Video
COVID-19 Testing Process
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Vivalytic Overview
SARS-CoV-2 Rapid Test
SARS-CoV-2 Pooling Test
Vivalytic Test Menu
Vivalytic | SARS-CoV-2 Pooling Test

Vivalytic | SARS-CoV-2 Pooling Test
–
Detecting SARS-CoV-2 (COVID-19)
Detecting SARS-CoV-2 (COVID-19) from up to 5 Pooled Samples Simultaneously
Clinical Significance
SARS-CoV-2 Pooling (COVID-19) is a rapid real time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care. This enables the patient to take the recommended safety precautions without delay. The pooling could be done at the level of a ward, medical speciality, social bubble, or group of colleagues. It has potential for use in other settings, such as pre-operative screening, schools and universities, prisons, nursing homes, primary care, and large workplaces. Sample pooling allows more people to be tested quickly using fewer testing resources.
In collaboration with Bosch, we are proud to release not only a rapid testing solution for the detection of SARS-CoV-2 (COVID-19) but an accelerated mass testing solution to effectively and efficiently monitor and detect viral infection from the offset with an aim of minimising the rise in infections globally. The new SARS-CoV-2 pooling test will allow users to test up to 160 samples a day and has sensitivity of 98% and a specificity of 100% – a world’s first!* Highly sensitive assays allow for laboratories to accurately detect low positive samples, enabling for effective identification of positive COVID-19 cases in a timely manner.
* Detailed information on the determination of analytical performance can be found in instructions for use provided with SARS-CoV-2 pooling kit.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab
Sample Volume: 150 μL per-patient sample (If
less than 5 patient samples, supplement the remaining
volume with eNAT solution)
Detection Method: Real-Time PCR
Time to result: 44 minutes
| Detectable Pathogens |
|---|
| SARS-CoV-2 (E gene sequence) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
SARS-CoV-2 Rapid Test
Vivalytic
Vivalytic Test Menu
Vivalytic | Sexually Transmitted Infection Array

Vivalytic | Sexually Transmitted Infection Array
–
1 Sample | 1 Test | 10 Infections
Comprehensive Sexual Health Profile
Clinical Significance
The Sexually Transmitted Infection (STI) array is the broadest multiplex cartridge-based STI test on the market simultaneously detecting 7 bacterial, 2 viral and 1 protozoan infection for a comprehensive sexual health profile.
Globally, each day over 1 million people are exposed to an STI, many of who will not know they have an infection as many STI’s are asymptomatic whilst some individuals will display similar or overlapping symptoms, therefore co-infections may remain undiagnosed. Designed to offer a complete sexual health profile with an aim of prevention and control, the Vivalytic STI array can be used to diagnose existing infections whilst any identifying co-infections.
To find out more about the global economic burden of STIs and the impact of the COVID-19 pandemic on sexual health, download our most recent whitepaper that presents issues such as AMR, the development of super-gonorrhoea, and the challenges faced in a social and public concept due to the COVID-19 pandemic.
Features
Sample Type: Swab or Urine (eNAT, Roche COBAS medium, or PBS)
Sample Volume: 300 μl
Detection Method: Randox Biochip Technology (end-point PCR)
Time to result: 2 hours 20 minutes
| Detectable Infections | |
|---|---|
| Chlamydia trachomatis (CT) | Herpes simplex virus 1 (HSV-1) |
| Neisseria gonorrhoeae (NG) | Herpes simplex virus 2 (HSV-2) |
| Trichomonas vaginalis (TV) | Haemophilus ducreyi (HD) |
| Mycoplasma genitalium (MG) | Mycoplasma hominis (MH) |
| Treponema pallidum (Syphilis) (TP) | Ureaplasma urealyticum (UU) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
Vivalytic Test Cartridges
Vivalytic
Vivalytic Test Menu
New 39-minute COVID test available on Randox-Bosch Vivalytic
02 October 2020
New 39-minute COVID test available on Randox-Bosch Vivalytic
- The world’s fastest PCR based SARS-CoV-2 test for the point of care delivers reliable results in 39 minutes.
- Has a sensitivity of 98 percent and a specificity of 100 percent.
- Simultaneous testing of five people with one cartridge by pooling will be available from early October.
- Work is in progress to further reduce time to result.
A rapid new coronavirus test, which provides results for Covid-19 in just 39 minutes, is now available on the Vivalytic, a point of care platform brought to market by Randox Laboratories and Bosch.
The test for detection of the SARS-CoV-2 pathogen, is currently the fastest PCR test (the gold standard of test methods) worldwide, and is predestined for decentralized use in mobile test centres at service stations or in airports, so that people who take the test can obtain a reliable result while at the testing site.
Available now in Europe, the CE-approved test, which has a sensitivity of 98 percent and a specificity of 100 percent, helps avoid time in quarantine, relieve laboratories, and make travel and work safer again.
“Rapid and accurate testing plays a crucial role in identifying cases of Covid-19 – to contain any outbreaks and limit the spread of the virus,” says Dr. Heather McMillan, Molecular R&D Manager at Randox Biosciences.
“This new rapid test will be a game-changer in the coronavirus testing landscape by allowing patients to receive their results at the point of care faster than ever before.”
Randox and Bosch launched the first rapid test for the Vivalytic analyser at the end of March, after just six weeks’ development.
As a multiplex test, it simultaneously checks samples for the SARS-CoV-2 virus as well as nine other respiratory diseases in two and a half hours, whereas the new accelerated test is exclusively for SARS-CoV-2.
“With our different coronavirus tests and variable analysis strategies, we open up a range of testing scenarios with a Vivalytic device – from screening all the way to supporting differential diagnosis for similar symptoms,” says Marc Meier, president of Bosch Healthcare Solutions GmbH.
And development work for Covid tests on the Vivalytic is ongoing: as of early October 2020, by pooling samples together it will be possible to simultaneously evaluate five samples in one test cartridge and at a comparable speed – a world first.
This will increase available testing capacity, by enabling fully automated processing of more than 160 samples a day using a Vivalytic device.
Key Benefits of SARS-CoV-2 test on Vivalytic point of care platform
The advantages of the rapid SARS-CoV-2 test on Vivalytic lie not only in speedy analysis, but also in ease of use. A sample is taken from the nose or throat using a swab, and placed in the test cartridge. Then the cartridge, which contains all the reagents required for the test, is inserted into the Vivalytic device for automated analysis.
- Turnaround time of 39 mins from sample entry to result.
- The SARS-CoV-2 rapid test has recently received CE marking.
- The SARS-CoV-2 pooling test can run up to 5 samples on-board one single cartridge.
- Easy 4-step user-friendly process from sample entry to result. Minimal training required.
- Detection from real-time PCR from Nasopharyngeal and/or Oropharyngeal swab.
- Suitable for use in any laboratory and non-laboratory settings.
The development of the new Vivalytic PCR singleplex test is part of a research and development project funded by the German Federal Ministry of Education and Research (BMBF).
For more information please contact marketing@randox.com
RESEARCH
BIOPHARMA
CLINICAL LAB
BIOREAGENTS
Vivalytic | Test Cartridges

Vivalytic | Cartridges
–
Powered by Biochip Technology
Simple & Accurate POC Molecular Diagnostics
Vivalytic cartridges are compact, technologically advanced Molecular Diagnostic tests utilising micro-fluidics to enable simple and accurate diagnostic testing. Vivalytic cartridges are powered by a variety of technologies, dependent upon the test application. High-Plex and Low-Plex tests can be analysed on the Vivalytic. High-Plex tests utilise Randox patented Biochip Array Technology, enabling end-point qualitative PCR and providing multiple test results from each sample. Low-Plex tests are based on a variety of detection methods including real-time qualitative PCR and melting curve analysis.
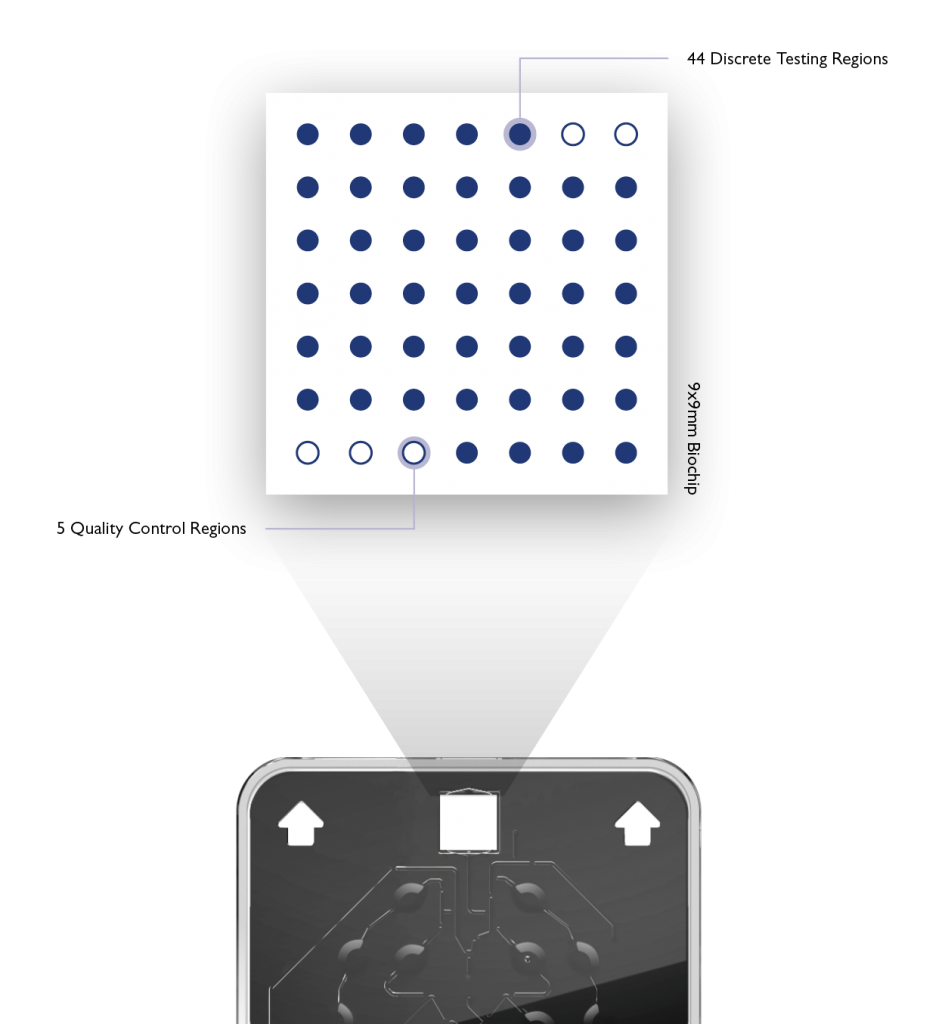
Randox patented Biochip Technology allows simultaneous detection of multiple targets from a single patient sample. The biochip detection system is based on a chemiluminescent signal, this is the emission of light, without heat, as a result of a chemical reaction. Each biochip is prefabricated with spatially discrete testing regions (DTR’s).
Each DTR represents an individual test. Each DTR can be occupied with oligonucleotides specific to a pathogen or target of interest. The High-Plex capabilities of Biochip Technology eliminates the need to run multiple time consuming and sample intensive assays.
An enzyme is used to catalyse the chemical reaction of the biochip which generates the chemiluminescent signal. The light emitted from the chemiluminescent reaction that takes place in each DTR is simultaneously detected and quantified using a Charge – Coupled Device (CCD) Camera. This CCD Camera simultaneously records the light emission from all the DTRs on each biochip. The Vivalytic automatically generates a result report for all targets.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
SARS-CoV-2 Rapid Test
VRI Array
Vivalytic
Vivalytic Test Menu
Vivalytic | SARS-CoV-2 Rapid 39 Minute Test

Vivalytic | SARS-CoV-2 Rapid 39 Minute Test
–
Detecting SARS-CoV-2 (COVID-19)
Rapidly Detecting SARS-CoV-2 (COVID-19)
Clinical Significance
SARS-CoV-2 (COVID-19) is a rapid real time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care. This enables the patient to take the recommended safety precautions without delay.
In collaboration with Bosch, we are proud to release not only a rapid testing solution for the detection of SARS-CoV-2 (COVID-19) but an accelerated mass testing solution to effectively and efficiently monitor and detect viral infection from the offset with an aim of minimising the rise in infections globally. The new SARS-CoV-2 pooling test will allow users to test up to 160 samples a day and has sensitivity of 98% and a specificity of 100% – a world’s first!*
* Detailed information on the determination of analytical performance can be found in instructions for use provided with SARS-CoV-2 kit.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab (eNAT)
Sample Volume: 300 μl
Detection Method: Real-Time PCR
Time to result: 39 minutes
| Detectable Virus |
|---|
| SARS-CoV-2 (E gene sequence) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
SARS-CoV-2 Pooling Test
Vivalytic
Vivalytic Test Menu
Vivalytic | The All in One Molecular Solution

Vivalytic | All In One Molecular Solution
–
Making a Point to Care
Molecular Diagnostics at the Point of Care
Consolidate the Full Molecular Workflow
Supporting Team GB through the Paris Olympics
The Vivalytic has supported Team GB by providing comprehensive testing for a wide range of respiratory infections from a single sample. This allows for targeted management of athletes’ health, ensuring precise treatment and quick recovery.
Regular and rapid testing with the Vivalytic helped maintain athletes in peak physical and mental condition by ensuring infection was detected and treated at the earliest opportunity. Check out the video to the right to learn more about how Vivalytic helped prepare and support Team GB.
“It’s important we know the pathogen driving the disease process to be able to target management appropriately“
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
VRI Array
SARS-CoV-2 Rapid Test
SARS-CoV-2 Pooling Test
Vivalytic Test Menu
Vivalytic | Viral Respiratory Infection Array

Vivalytic | 10-Plex Viral Respiratory Infection Array
–
Detect 10 viral respiratory infections in 2 hours 30 minutes
Research Use Only
Detecting SARS-CoV-2 (COVID-19)
Clinical Significance
The Viral Respiratory Tract Infections (VRI) test offers a rapid and comprehensive solution, capable of detecting 10 different viral respiratory infections, including SARS-CoV-2, within a remarkably short timeframe of 2 hours and 30 minutes.
This extensive panel equips healthcare providers with the ability to swiftly identify potential co-infections, thereby enabling them to make more informed treatment decisions for patients grappling with respiratory illnesses. The expedited detection of multiple viruses not only aids in timely intervention but also holds the promise of reducing unnecessary antibiotic usage, consequently leading to improved patient outcomes and more effective management of respiratory conditions.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab (eNAT)
Sample Volume: 300 μl
Detection Method: Randox Biochip Technology (end-point PCR)
Time to result: 2 hours 30 minutes
| Detectable Viruses | ||
|---|---|---|
| SARS-CoV-2 (COVID-19) | Sarbecovirus (SARS, SARS like, SARS-CoV-2) | Influenza A |
| Coronavirus 229E/NL63 | Adenovirus A/B/C/D/E | Influenza B |
| Coronavirus OC43/HKUI | Enterovirus A/B/C/D / Rhinovirus A/B/C | Respiratory Syncytial Virus A/B (RSV) |
| Middle East Respiratory Syndrome Coronavirus (MERS-CoV) | Adenovirus A/B/C/D/E | |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
SARS-CoV-2 Rapid Test
SARS-CoV-2 Pooling Test
Vivalytic
Vivalytic Test Menu
Norbrook partners with Randox on staff screening programme for COVID-19
Pharmaceuticals company Norbrook Laboratories has revealed that it will be the first company in Northern Ireland to install an innovative COVID-19 testing platform for on-site staff screening.
The Newry-based firm, which specialises in the development of pharmaceuticals for the veterinary industry, will begin testing for its workforce in the coming weeks, to provide reassurance and peace of mind for colleagues and for their families, without impacting on the public health need.
The COVID-19 screening programme at Norbrook is being facilitated by diagnostic technology from global diagnostics company Randox, one of the partners within the national COVID-19 testing programme.
David Ferguson, Managing Director at Randox Food Diagnostics explained;
“Collectively we are all working towards a timely return to a more normal society, which will see companies reopening and people returning to work.
“To facilitate this recovery of the economy, without compromising the health of workers or of the wider general public, workplaces have a responsibility to provide a safe working environment.
“We know that the health of Norbrook’s staff is their priority and as such it is great to see them taking a proactive approach to testing.”
The COVID-19 screening programme at Norbrook is the first in Northern Ireland being facilitated by an innovative testing platform capable of processing results in 2hours 30 mins.
The technology, named the Vivalytic, can screen for SARS-CoV-2, the virus that causes COVID-19, as well as a range of other viral and bacterial infections including Influenza A and B, Pneumonia and other coronaviruses.
David added;
“Randox is fully committed to supporting the national effort to fight COVID-19 by testing at scale, and as we continue to work alongside the government and ramp up our testing capabilities, we welcome the fact that other organisations are also adopting our innovative COVID-19 testing technologies to address their own particular testing needs.
“The Vivalytic, which provides high quality molecular testing for COVID-19, on-site and without the need for laboratory experience, is a unique space-saving, hygienic solution for personnel COVID-19 testing in any setting and will help a wide variety of industries get back to business by ensuring the highest level of safety.”
Denise Collins, Norbrook Human Resources Director, concluded;
“We’re proud to be working with a company like Randox which has such vast experience in the diagnostics industry and was subsequently able to respond so quickly to the COVID-19 pandemic.
“That this world-leading technology is available from a Northern Ireland-headquartered company presents a unique opportunity for workplaces here to quickly and easily implement a staff screening programme, and at the same time demonstrate a high standard of duty of care that should be shown by employers to their employees.”
For further information about the Vivalytic please email marketing@randox.com
Want to know more about Randox?
Contact us or visit our homepage to view more.
Our Randox Products and Services
REAGENTS
RX SERIES
ACUSERA
BIOCHIP
Point of care coronavirus test from Randox and Bosch will launch in April 2020
26 March 2020
Vivalytic Viral Respiratory Tract Infection Array Detecting SARS-CoV-2 (COVID-19)
A game-changing point of care coronavirus test from global diagnostics company Randox Laboratories and leading technology manufacturer Bosch Healthcare Solutions will launch in April 2020.
The Vivalytic Viral Respiratory Tract Infection (VRI) Array can identify SARS-CoV-2 (COVID-19) and differentiate it from nine other respiratory infections with similar symptoms, including influenza and all known coronaviruses.
This provides a more comprehensive respiratory screening which enables precise and informed treatment decisions to be made.
Dr. Heather McMillan, Molecular R&D Manager at Randox Biosciences, commented;
“This array focuses not only on the identification of the novel coronavirus strain that causes COVID-19, but also nine other respiratory infection targets simultaneously, including Influenza A and B, Sarbecovirus and MERS. The aim is to diagnose patients fast and accurately from a single genetic sample, maximising containment of the virus whilst minimising the spread. With the development of this array onto a fully automated platform, patients can be diagnosed rapidly at point of care locations globally such as pharmacies and doctor surgeries.”
The Viral Respiratory Tract Infection Array is one of the world’s first multiplex molecular diagnostic tests meeting the COVID-19 testing recommendations of the World Health Organisation (WHO) and the Centres for Disease Control (CDC).
The target genes for COVID-19 being used on the VRI array represent conserved regions of the genome which have been chosen for their high sensitivity and specificity.
Marc Meier, Managing Director of Bosch Healthcare Solutions, a subsidiary of Bosch Group, said:
“We are enthusiastic about partnering with Randox to offer their assay technology on the Vivalytic platform. The core competencies of Bosch in automation, miniaturization, sensor technology and connectivity are complemented by Randox’s expertise in developing excellent biocontent for a wide range of assays and commercializing innovative diagnostic solutions.”
The new VRI test will be conducted on Vivalytic a point of care platform brought to the market by Randox Laboratories and Bosch. The Vivalytic system is a fully-automated, cartridge-based platform capable of both Hi-Plex and Lo-Plex testing. The cartridges are fully-sealed which minimises the risk of contamination, require room temperature storage (space-saving), contain all the reagents on-board the cartridge and utilises end point PCR. The test only requires a single nasal swab from the patient and an easy four step process to be carried out by the user to run the patient sample.
Key Benefits of Vivalytic | Point of Care Platform
- Fully-automated
- User friendly
- No laboratory training required
- 4 step work flow
- Minimal contamination risk – fully contained system
- No peripherals required – hygienic
- Touchscreen
- Connects to LIMS (Laboratory Information Management System)
- Sample Type: Nasopharyngeal Swab
- Sample Volume: 300µl clinical sample
For more information or to arrange interviews, please contact info@randoxbiosciences.com



















