Solutions
keyboard_arrow_downServices
keyboard_arrow_downSupport & Resources
keyboard_arrow_downCompany
keyboard_arrow_downContact
QCMD is a world leading Molecular EQA / Proficiency Testing scheme specialising in infectious disease testing.
Benefits
High Level of Participation
Over 15,000 participants in over 120 countries, ensuring peer group numbers are maximised.
Frequent Reporting
A variety of options are available. Choose the number of EQA challenges that best suit your requirements.
Extensive EQA Offering
Boasting the largest selection of molecular EQA programmes for infectious disease testing, you are sure to find what you're looking for.
Online EQA Data Management
ITEMS provides an online tool to easily manage all EQA activities including result submission and access to EQA reports.
International Accreditation
Programmes accredited to ISO 17043 Conformity assessment – General requirements for the competence of proficiency testing providers.
Comprehensive Reports
Receive individual reports for each EQA challenge, allowing visual performance assessment and a final supplementary report at the end of the cycle.
Get in touch to discover more
To find out more about QCMD EQA or to get in touch with your local Randox Represnetative, enquire now.
EQA Schemes
- Bloodborne Virus
- Central Nervouse System
- Congenital Infections
- Drug Resistance
- Exotic/Emerging Diseases
- Gastrointestinal Diseases
- Immunocompromised Associated Diseases
- Multiple Pathogen/Syndromic
- Respiratory Diseases
- Sexually Transmitted Infections
- Transplant Associated Diseases
- Typing
- Other
- Pilot Studies
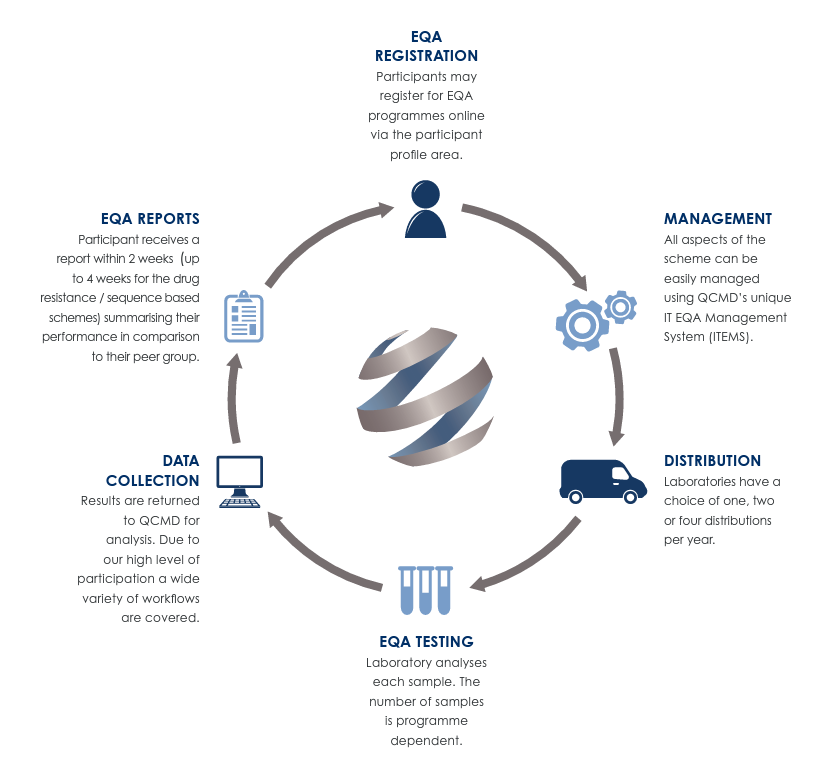
How it Works
Reports & Feedback
After the close of the results return phase, EQA participants will receive an individual report outlining their performance relative to their method and technology groups. A supplementary report may be commissioned - this includes any additional relevant information regarding the annual EQA distribution, as well as scientific expert commentary and feedback on the overall results within that distribution.
*Randox are authorised by QCMD to provide the QCMD EQA schemes under a strategic global partnership. The EQA design, composition, data analysis & reporting remain the responsibility of QCMD. Please refer to specific geographical regions for further details on availability.