Solutions
keyboard_arrow_downServices
keyboard_arrow_downSupport & Resources
keyboard_arrow_downCompany
keyboard_arrow_downContact
Quality Control solutions for Molecular Infectious Disease testing designed to meet the demands of today's molecular diagnostics laboratory.
Our range comprises hundreds of characterised viral, bacterial and fungal targets covering a wide range of diseases.
Benefits
Whole Pathogen
Whole genome controls that mimic samples, monitoring testing from extraction to detection.
True Third Party
Independent, unbiased assessment of performance, supporting ISO 15189 compliance.
Liquid for Ease-of-Use
Liquid frozen samples, reducing preparation time and manual handling.
Traceability
All controls traceable to international reference material and/or standards per ISO 17511.
Target Pathogen Provided
Covering the detection of pathogens associated with a wide range of diseases.
Flexible
Supporting routine assay monitoring, Limit of Detection (LOD), Limit of Quantitation (LOQ) and sensitivity
Get in touch to discover more
To find our more about Qnostics Controls or to get in touch with your local Randox Representative, enquire now.
Qnostics Control Range
Discover the range below
Blood Borne Viruses
Central Nervous System
Drug Resistance
Gastrointestinal Infections
Respiratory Infections
Sexually Transmitted Infections
Transplant Associated Diseases
Other
Solutions
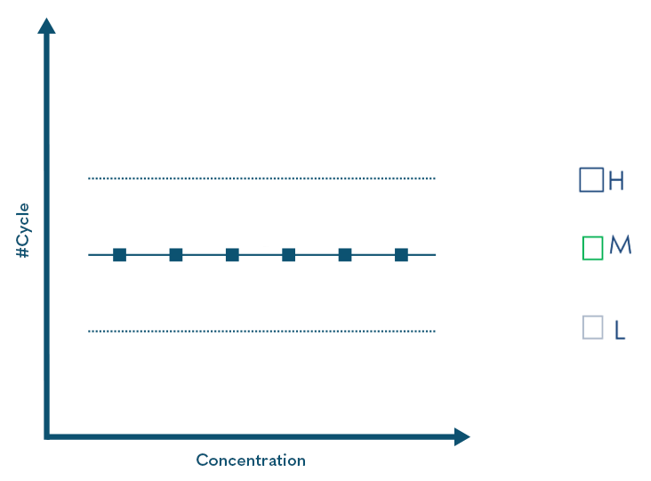
Q Controls
Qnostics' 'Q' Controls are positive run controls intended to help laboratories monitor their molecular diagnostic assay on a run-to-run basis within customer derived limits. These third-party independent external controls will ensure that assay drift is detected, monitored and managed allowing the laboratory to continue to provide accurate and reliable results. They also help support a laboratory's regulatory requirements under the standard ISO 15189.